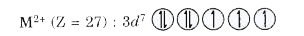Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise NCERT FILE (TEXTBOOK EXERCISE)|38 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise NCERT FILE (EXEMPLAR PROBLEM (MULTIPLE CHOICE QUESTION TYPE -I))|21 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise CONCEPTUAL QUESTION|49 VideosCHEMISTRY IN EVERYDAY LIFE
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|20 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-D AND F-BLOCK ELEMENTS-NCERT FILE (INTEXT QUESTION)
- Silver atom has completely filled d orbitals (4d^(10)) in its ground s...
Text Solution
|
- In the series Sc(Z=21) to Zn(Z=30) the enthalpy of atomisation of zinc...
Text Solution
|
- Which of the 3d series of the transition metals exhibits the largest n...
Text Solution
|
- The E^(0)(M^(2+)//M) value for copper is positive (+0.34V). What is po...
Text Solution
|
- How would you account for the irregular of ionisation enthalpies (firs...
Text Solution
|
- Why is the highest oxidation state of a metal exhibited in its oxide o...
Text Solution
|
- Which is a stronger reducing agent Cr^(2+) or Fe^(2+) and why?
Text Solution
|
- Calculate the spin only magentic moment of M^(2+) ion (Z=27).
Text Solution
|
- Explain why Cu^+ ion is not stable in aqueous solution ?
Text Solution
|
- Actinoid contraction is greater from element to element than lanthanoi...
Text Solution
|