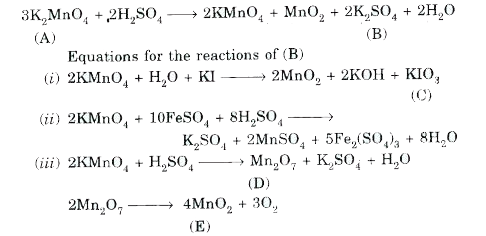Text Solution
Verified by Experts
Topper's Solved these Questions
D AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise COMPETITION FILE (MULTIPLE CHOICE QUESTION ((A) MULTIPLE CHOICE QUESTION WITH ONLY ONE CORRECT ANSWER))|35 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise COMPETITION FILE (MULTIPLE CHOICE QUESTION ((B) MULTIPLE CHOICE QUESTION FROM COMPETITIVE EXAMINATION))|71 VideosD AND F-BLOCK ELEMENTS
MODERN PUBLICATION|Exercise REVISION EXERCISES ( LONG ANSWER QUESTION)|24 VideosCHEMISTRY IN EVERYDAY LIFE
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|13 VideosELECTROCHEMISTRY
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|20 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-D AND F-BLOCK ELEMENTS-HIGHER ORDER THINKING SKILLS
- Give reasons in two or three sentences only for the following "The s...
Text Solution
|
- The second ionisation enthalpies of both Cr and Cu are higher than tho...
Text Solution
|
- First ionisation energy of copper is higher than those of alkali metal...
Text Solution
|
- Mercurous ion is written as Hg(2)^(2+) whereas cuprous ion is written ...
Text Solution
|
- In the titration of Fe^(2+) ions with KMnO4 in acidic medium, why is ...
Text Solution
|
- Why hydrated copper sulphate is blue while anhydrous copper sulphate i...
Text Solution
|
- Which of two : cuprous chloride or cupric chloride is coloured and why...
Text Solution
|
- A mixed oxide of iron and chromium, FeO.Cr2O3 is fused with sodium car...
Text Solution
|
- Calculate the magnetic moment (spin only) of manganese in K4 [Mn(NCS)...
Text Solution
|
- HgCl2 and SnCl2 cannot coexist together in an aqueous solution.
Text Solution
|
- In the transition series, starting from lanthanum La(Z=57), the next e...
Text Solution
|
- The 4d and 5d series of transition metals have more frequency metal-me...
Text Solution
|
- Between Na^(+) and Ag^(+) which is stronger Lewis acid and why?
Text Solution
|
- Identify A to E. Pyrolusite on heating with KOH in the presence of air...
Text Solution
|
- When a white crystalline compound X is heated with K2Cr2O7 and concent...
Text Solution
|
- (a) A blackish brown coloured solid 'A' when fused with an alkali meta...
Text Solution
|