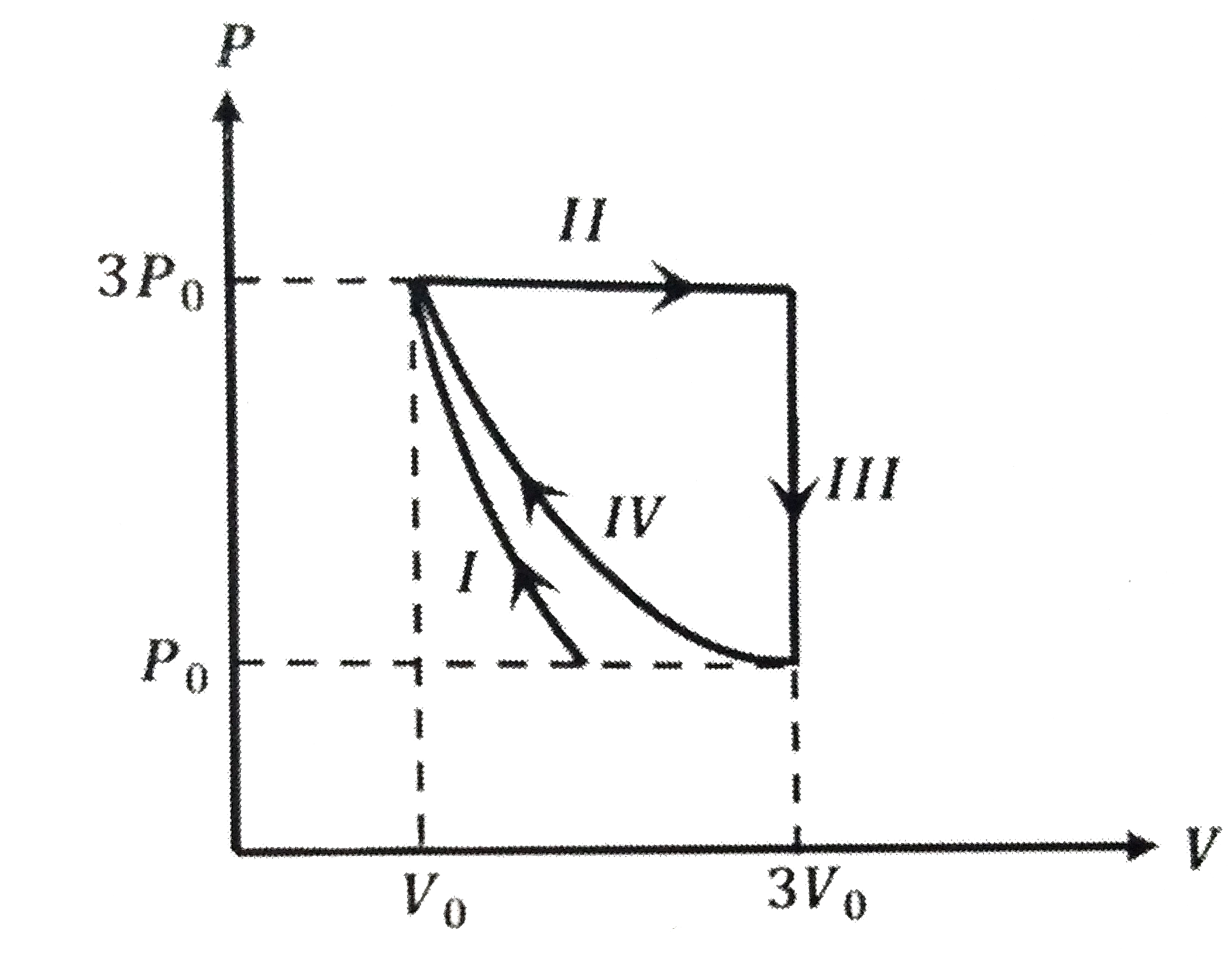
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
JEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise MULTIPLE CHOICE QUESTIONS|22 VideosJEE ADVANCED
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION-III (Comprehension Type )|3 VideosJEE (ADVANCE) 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION 3|6 VideosJEE ADVANCED 2020
JEE ADVANCED PREVIOUS YEAR|Exercise SECTION-3|6 Videos
Similar Questions
Explore conceptually related problems