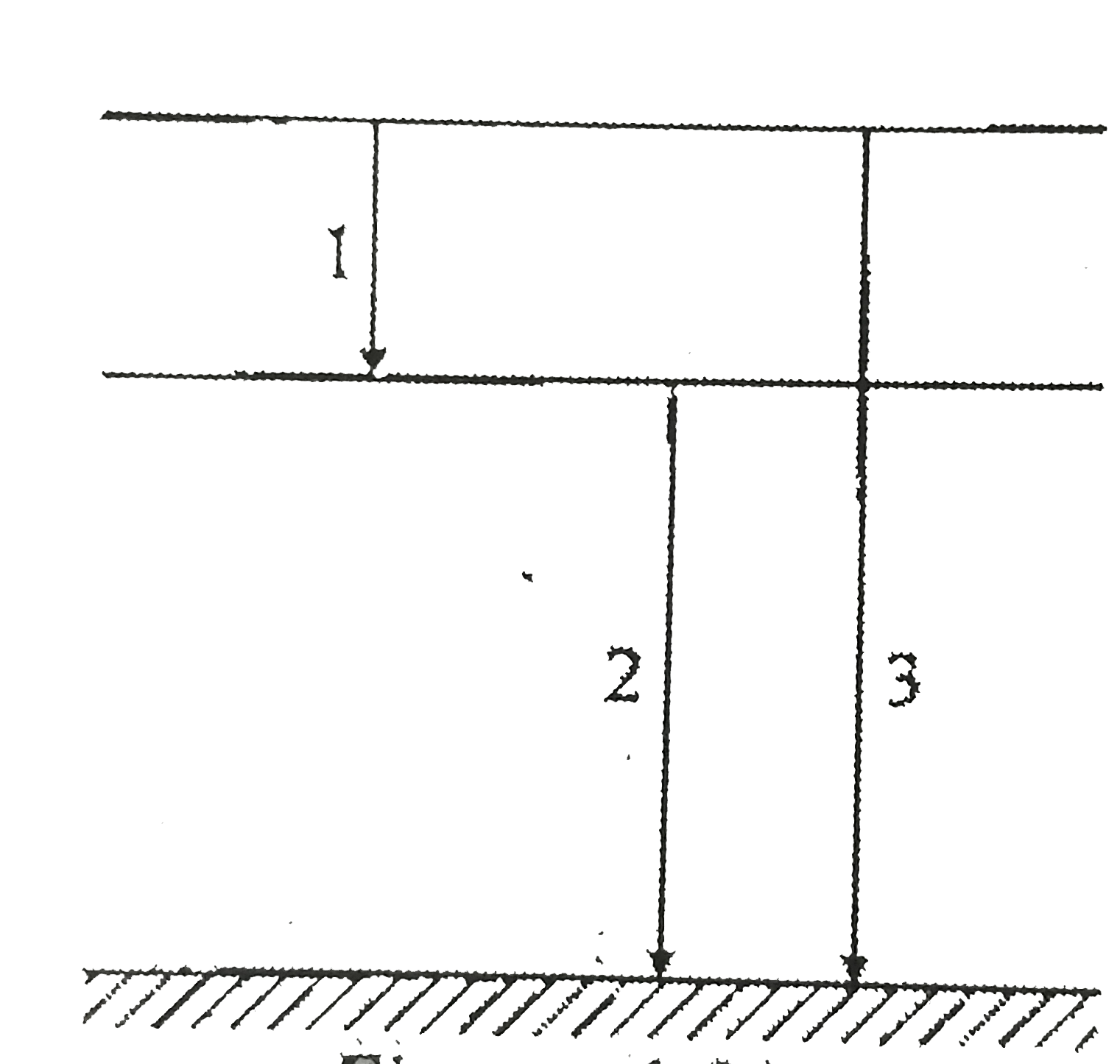
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN ATOM
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (Linked Comprehension)|9 VideosHYDROGEN ATOM
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS(Matrix - Match)|8 VideosHYDROGEN ATOM
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (Single Correct Choice Type)|51 VideosHEAT-MEASUREMENT AND TRANSFER
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS( INTEGER TYPE)|5 VideosINTERFERENCE AND DIFFRACTION
RESNICK AND HALLIDAY|Exercise PRACTICE QUESTIONS (Integer Type)|6 Videos
Similar Questions
Explore conceptually related problems
RESNICK AND HALLIDAY-HYDROGEN ATOM-PRACTICE QUESTIONS (More than One Correct Choice Type)
- An electron is revolving around a nucleus of hydrogen atom in the firs...
Text Solution
|
- According to Bohr's theory, hydrogen atom for an electron in the nth p...
Text Solution
|
- In an electron transition inside a hydrogen atom, orbital angular mome...
Text Solution
|
- Whenever a hydrogen atom emits a photon in the Balmer series
Text Solution
|
- An electron is excited from a lower energy state to a higher energy st...
Text Solution
|
- An electron in hydrogen atom first jumps from second excited state to ...
Text Solution
|
- A neutron collies head-on with a stationary hydrogen atom in ground st...
Text Solution
|
- The wavelengths and frequencies of photons in transition 1,2 and 3 for...
Text Solution
|
- A particular hydrogen like atom has its ground state Binding energy = ...
Text Solution
|
- In the Bohr model of the hydrogen atom, let R, v and E represent the r...
Text Solution
|
- When Z is doubled in an atom, which of the following statements are co...
Text Solution
|
- If electron of hydrogen atom is replaced by another particle of same c...
Text Solution
|
- If the potential energy is taken zero at ground state of hydrogen atom...
Text Solution
|
- A beam of ultraviolet light of all wavelength passes through hydrogen ...
Text Solution
|
- A free hydrogen atom in ground state is at rest. A neutron of kinetic ...
Text Solution
|