Text Solution
Verified by Experts
Topper's Solved these Questions
CARBON AND ITS COMPOUNDS
VK GLOBAL PUBLICATION|Exercise HOTS (Higher Order Thinking Skills)|13 VideosCARBON AND ITS COMPOUNDS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (Very Short Answer Questions)|6 VideosCARBON AND ITS COMPOUNDS
VK GLOBAL PUBLICATION|Exercise SHORT ANSWER QUESTIONS-II|18 VideosACIDS, BASES AND SALTS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE ( Long Answer Questions (5 mark))|4 VideosCHEMICAL REACTIONS AND EQUATIONS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (LONG ANSWER QUESTIONS)|2 Videos
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-CARBON AND ITS COMPOUNDS-LONG ANSWER QUESTIONS
- Both soap and detergent are some type of salts. What is the difference...
Text Solution
|
- What happens when (i) ethanol burns in air. (ii) ethanol reacts wi...
Text Solution
|
- (a) What are hydrocarbons ? Give examples. (b) Give the structural d...
Text Solution
|
- Explain why carbon forms compounds mainly by covalent bond. Explain i...
Text Solution
|
- A compound C (molecular formula, C(2)H(4)O(2)) reacts with Na metal to...
Text Solution
|
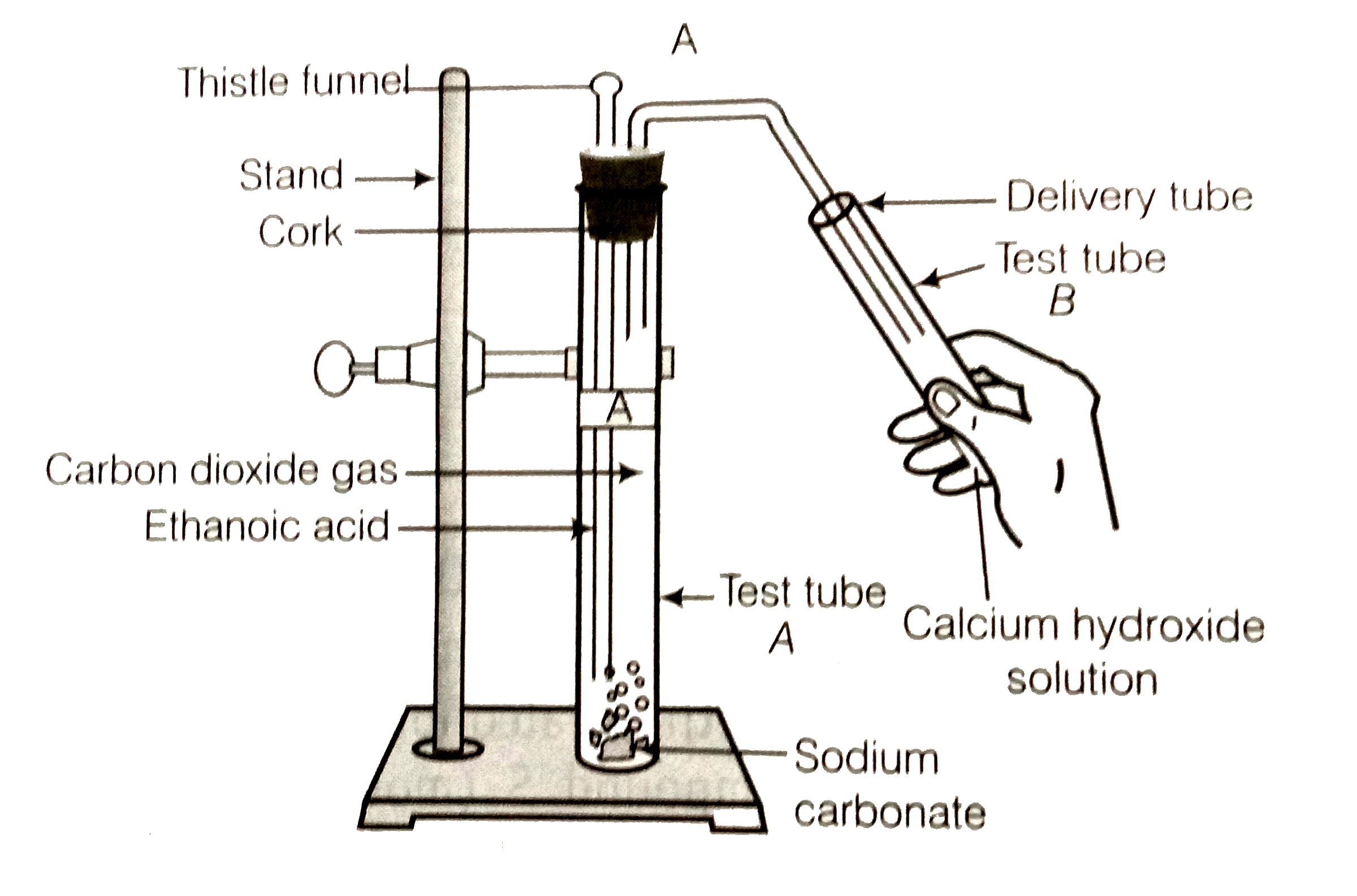
- Look at the figure and answer the following questions. (a) What...
Text Solution
|
- A salt X is formed and a gas is evolved when ethanoic acid reacts with...
Text Solution
|
- (a) Give a chemical test to distinguish between saturated and unsatura...
Text Solution
|
- Elements forming ionic compounds attain noble gas electronic configura...
Text Solution
|
- (a) You have three unlabelled test tubes containing ethanol, ethanoic ...
Text Solution
|