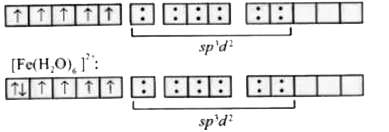
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (Category 1: Single Option Correct Type)|6 VideosCOORDINATION COMPOUNDS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 3; ONE OR MORE THAN ONE OPTION CORRECT TYPE(|3 VideosCOORDINATION COMPOUNDS
MTG-WBJEE|Exercise WBJEE/WORKOUT (Category 2: Single Option Correct Type)|13 VideosCHEMISTRY OF NON-METALLIC ELEMENTS AND THEIR COMPOUNDS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS(CATEGORY 3 : One or More Option Correct Type)|1 VideosENVIRONMENTAL CHEMISTRY
MTG-WBJEE|Exercise WB JEE Previous Years Questions ( CATEGORY 1 : Single Option Correct Type )|2 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-COORDINATION COMPOUNDS-WBJEE/WORKOUT (Category 3: One or More than one Option Correct Type)
- In which of the following cases, the complex ion formed will migrate t...
Text Solution
|
- Which of the following are outer orbital octahedral complexes?
Text Solution
|
- Identify the complexes which are expected to be coloured.
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Which of the following statements are false?
Text Solution
|
- Which of the following involves sp^(3) hybridisation and are tetrahedr...
Text Solution
|
- Which of the following ions show higher spin only magnetic moment valu...
Text Solution
|