A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL DYNAMICS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 1 : Single Option Correct Type )(1 Mark)|4 VideosCHEMICAL DYNAMICS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 2: Single Option Correct Type )(2 Marks)|3 VideosCHEMICAL DYNAMICS
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 3 : One or More than One Option Correct Type)(2 Marks)|2 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
MTG-WBJEE|Exercise WB JEE Previous Years Questons (Category 2 : One or More than one Option Correct Type )|3 VideosCHEMICAL ENERGETICS
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (ONE OR MORE THAN ONE OPTION CORRECT TYPE )|2 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-CHEMICAL DYNAMICS -WB JEE WORKOUT (CATEGORY 2 : Single Option Correct Type)(2 Mark)
- For the reaction 2A+B rarr product, doubling the initial concentration...
Text Solution
|
- The half-life period, t(1//2) is related to the order n and initial co...
Text Solution
|
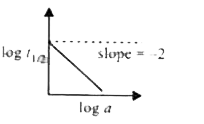
- A graph between log t(1//2) and log a (abscissa), a being the initial ...
Text Solution
|
- If a homogeneous catalytic reaction can take place through three alter...
Text Solution
|
- The rate expression for the reaction A((g)) + B((g)) rarr C((g))" is r...
Text Solution
|
- The rate constant (k') of one of the reaction is found to be double th...
Text Solution
|
- The rate of a gaseous reaction is given by the is expression k[A][B].I...
Text Solution
|
- The following graph shows how t(1//2) (half-life) of a reactant R chan...
Text Solution
|
- For a zero order reaction, A rarr Product with specific rate constant ...
Text Solution
|
- The time taken for 10% completion of a first order reaction is 20 mins...
Text Solution
|
- For a reversible chemical reaction where the forward process is exothe...
Text Solution
|
- The half-life period of radioactive element is 140 days. After 560 day...
Text Solution
|
- The bacterial growth follows the rate law,(dN)/(dt)=kN, where k is a c...
Text Solution
|
- At 380^(@)C, the half-life period for the first order decomposition of...
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following is/are correct regarding activation energy?
Text Solution
|
- The rate constants k1 and k2 for two different reactions are 10^(16).e...
Text Solution
|
- The activation energies of two reactions are E1" and "E2 (E1 gt E2). I...
Text Solution
|
- Arrhenius equation may be represented as
Text Solution
|
- Which of the following statements is/are incorrect regarding order of ...
Text Solution
|