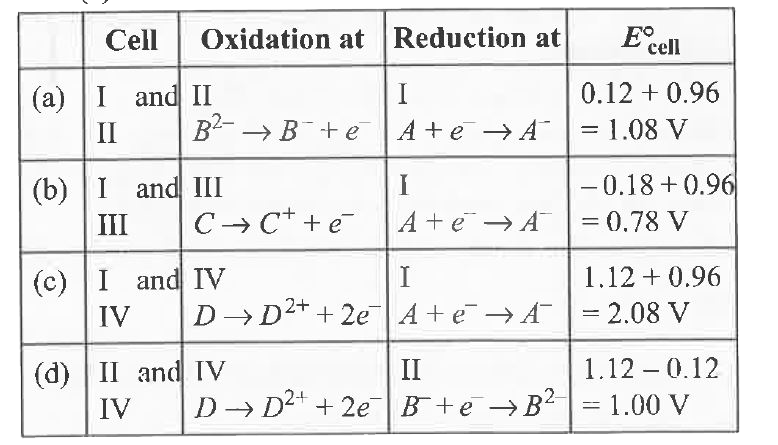
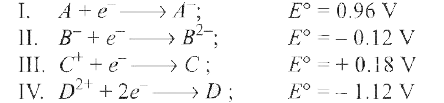A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX EQUILIBRIA
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS|5 VideosRADIOACTIVITY AND NUCLEAR CHEMISTRY
MTG-WBJEE|Exercise WB JEE PREVIOUS YEARS QUESTIONS (CATEGORY 2 : SINGLE OPTION CORRECT TYPE)|1 VideosSTATES OF MATTER (SOLIDS, LIQUIDS AND GASES)
MTG-WBJEE|Exercise WB JEE Previous Years Questions (CATEGORY 3; ONE OR MORE THAN ONE OPTION CORRECT TYPE(|1 Videos
Similar Questions
Explore conceptually related problems
MTG-WBJEE-REDOX EQUILIBRIA-WB JEE PREVIOUS YEARS QUESTIONS
- Consider the following half-cell reactions. What combination of t...
Text Solution
|
- The two half cell reactions of an electrochemical cell is given as ...
Text Solution
|
- In aqueous alkaline solution, two electron reduction of HO(2)^(-) give...
Text Solution
|
- given the standard half -cell potentials (E^@) of the followin...
Text Solution
|
- At temperature of 298 K the emf of the following electrochemical cell ...
Text Solution
|
- The formal potential of Fe^(3+)//Fe^(2+) in a sulphuric acid and phosp...
Text Solution
|