a. Total acid `= 50xx0.5xx2 = 50 mEq`.
Excess acid `= 60xx0.5 = 30 mEq`.
Acid used to neutralise `NH_(3) = 50 - 30 = 20 mEq`.
Perventage of `N = (1.44xx mEq. "of acid")/("Wt fo compd".)`
`= (1.4xx20)/(0.5) = 56%`
b. Caculate of emprical formula.
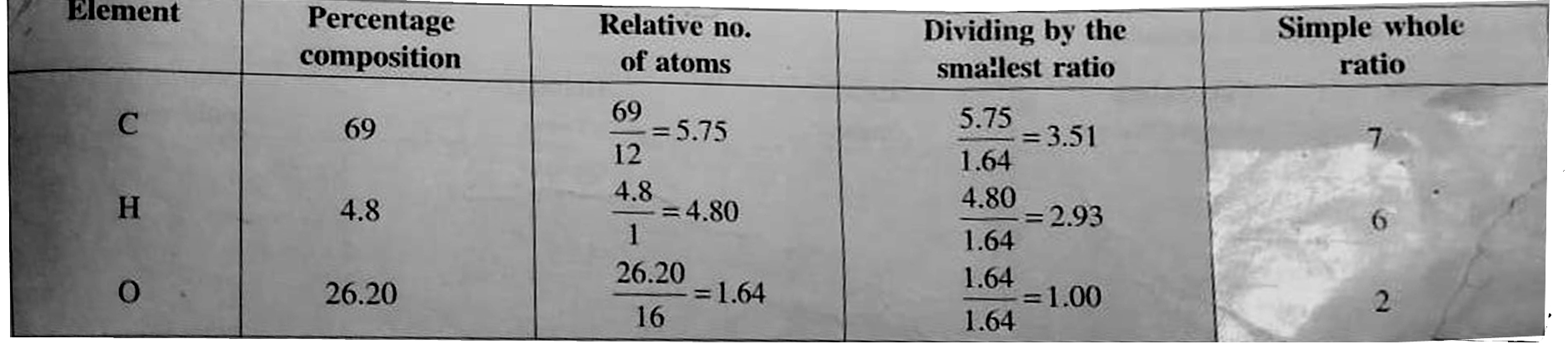
Mass of sample `= 0.20gm`, mass of `CO_(2)` formed `= 0.505gm` mass of water formed `= 0.864 gm`.
Percentage of carbon `= (12xx0.505xx100)/(44xx0.20) = 69%`
Percentage of hydrogen `= (2xx0.086xx100)/(18xx0.20) = 4.8%`
Percentage of oxygen `= 100-(69+4.8) = 26.20%`
Emprical formula `= C_(7)H_(6)O_(2)`
`40 gm` of `NaOH` would meutralise `1 mol` of a mononbasic acid
`NaOH = 25 xx (1)/(10) = 2.5 mEq. = 2.5xx10^(-3)xx40 = 0.1 gm`
`0.1gm` of `NaOH` neuralies `0.305 gm` acid
`40gm NaOH` neutralies `(0.305xx40)/(0.1) = 122gm`
Molecular mass of acid `= 122 gm mol^(-1)`
`n = ("Mol. formula mass")/("Empirical formula mass") = (122)/(122) = 1`
Molecular formula of acid `= (C_(7) H_(6) O_(2))_)1) = C_(7) H_(6) O_(2)`
c.
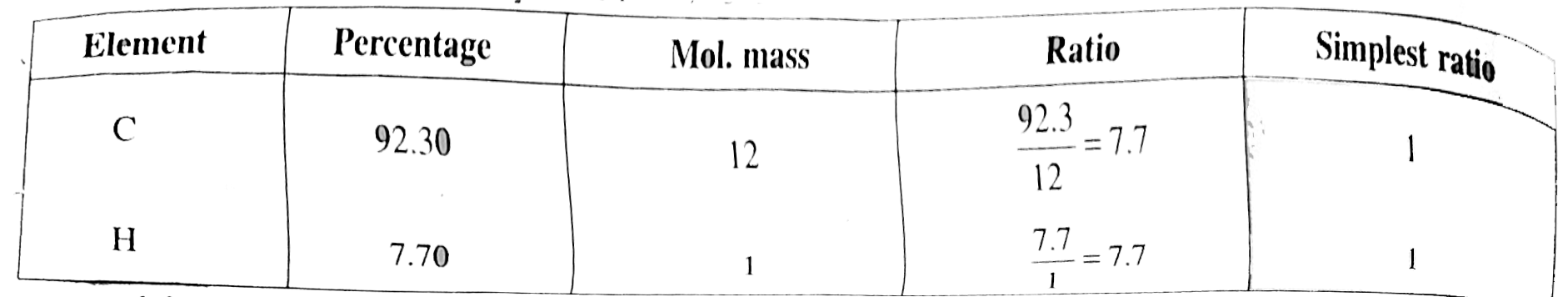
Empirical formula `= CH`
Mol mass of `(A) = 2xxV.D. = 2xx52 = 104`
`n = (104)/(13) = 8`
Molecule formula `= C_(8) H_(8)`
Decree of unsaturation `= [(2xx8+2) - 8]//2 = 5^(@)`
`5^(@) unsaturation shows that it is can aromatic compound,
`4^(@)` due to benzene ring.
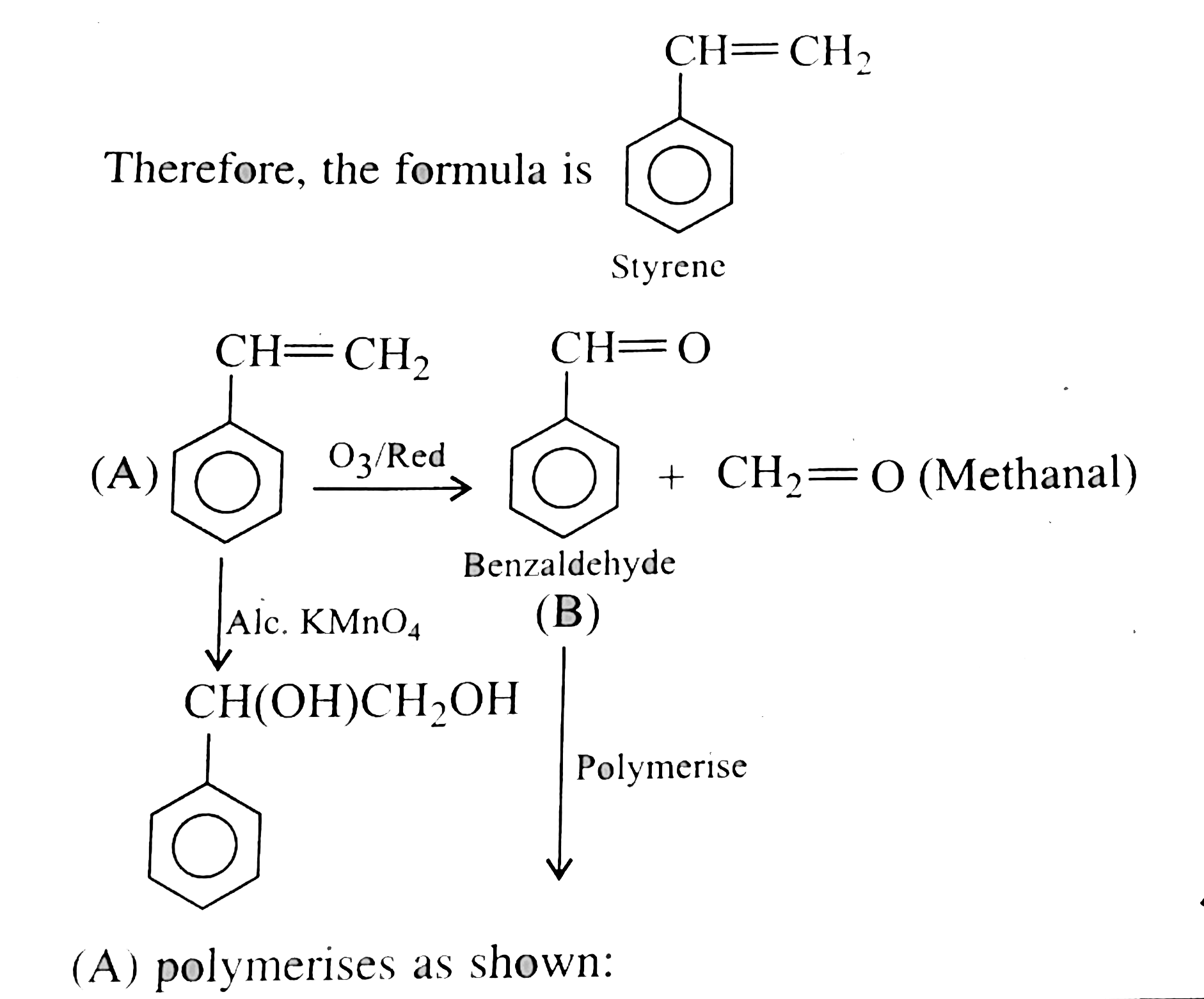
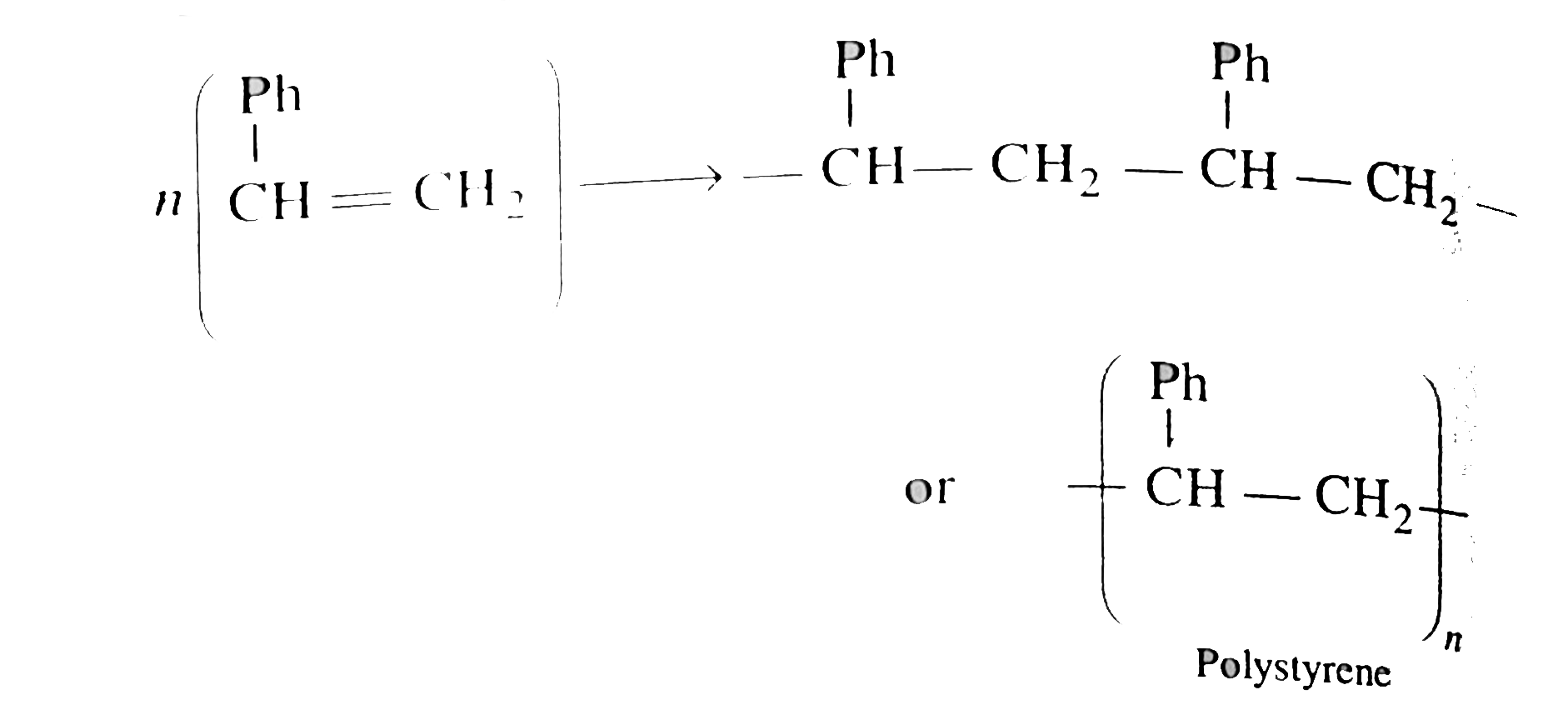
`(A)` plymerises as shwon:
`n(overset(Ph)overset(|)(CH)= CH_(2)) rarr - overset(Ph)overset(|)(CH) - CH_(2) - overset(Ph)overset(|)(CH) - CH_(2) -`
d. Mass of organic compound `= 0.26 gm`
Mass of `CO_(2) = 0.198 gm`
Mass of `H_(2)O = 0.1014 gm`
Percentage of `C = (12xx0.198xx100)/(44xx0.246) = 21.95%`
Percentage of `H = (2xx0.1014xx100)/(18xx0.246) = 4.58%`
Percentage of bromic `= (80xx0.638xx100)/(188xx037)`
`= 73.37%`

Emprical formula `= C_(2) H_(5) Br`, Molecular mass `= 109`
`n = 109//109 = 1`
Molecular formula `= (C_(2) H_(5)Br)_(1) = C_(2)H_(5)Br`




