A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise LECTURE SHEET( EXERCISE-I ) (MATRIX MATCHING TYPE QUESTIONS )|2 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise LECTURE SHEET( EXERCISE-I )(INTEGER TYPE QUESTIONS )|3 VideosCHEMICAL EQUILIBRIUM
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS|5 VideosCARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise CONVERSIONS|19 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL EQUILIBRIUM-LECTURE SHEET( EXERCISE-I )(LINKED COMPREHENSION TYPE QUESTIONS )
- In a 7.0 Levacuated chamber, 0.50 mol H(2) and 0.50 mol I(2) react at ...
Text Solution
|
- In a 7.0 Levacuated chamber, 0.50 mol H(2) and 0.50 mol I(2) react at ...
Text Solution
|
- In a 7.0 Levacuated chamber, 0.50 mol H(2) and 0.50 mol I(2) react at ...
Text Solution
|
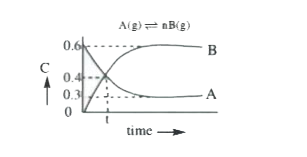
- The progress of the reaction A nB with time t is shown in Fig. Fro...
Text Solution
|
- The progress of the reaction A nB with time t is shown in Fig. From ...
Text Solution
|