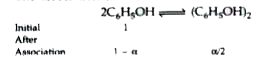Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTION
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION A (MULTIPLE CHOICE QUESTIONS))|7 VideosSOLUTION
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION D)|2 VideosSOLUTION
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS-I|32 VideosSOLID STATE
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|8 VideosSURFACE CHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST ( SECTION A)|7 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-SOLUTION-LONG ANSWER QUESTIONS-II
- A solution containing 30 g of non-volatile solute exactly in 90 g of w...
Text Solution
|
- Give reasons : (i) Aquatic species are more comfortable in cold water...
Text Solution
|
- (a) When 1.5 g of a non-volatile solute was dissolved in 90 g of benze...
Text Solution
|
- A 10% solution (by mass) of sucrose in water has a freezing point of 2...
Text Solution
|
- Define the following terms : (i) Molality (m) (ii) Abnormal molar mass...
Text Solution
|
- 30 g of urea (M = 60 g "mol"^(-1)) is dissolved in 846 g of water. Cal...
Text Solution
|
- Write two differences between ideal solutions and non-ideal solutions.
Text Solution
|
- Explain why a solution of chloroform and acetone shows negative deviat...
Text Solution
|
- Phenol associates in benzene to a certain extent to form a dimmer. A s...
Text Solution
|
- Derive the relationship between relative lowering of vapour pressure a...
Text Solution
|
- Define Azeotropes and explain briefly minimum boiling azeotrope by tak...
Text Solution
|
- The vapour pressures of pure liquids A and B are 450 mm and 700 mm of ...
Text Solution
|