Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (LONG ANSWER QUESTIONS-I (3 MARKS EACH))|27 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (LONG ANSWER QUESTIONS-II (5 MARKS EACH))|16 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (VERY SHORT ANSWER QUESTIONS (1 MARK EACH))|47 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|7 VideosEXAMINATION PAPER 2020
U-LIKE SERIES|Exercise SECTION D|15 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-ELECTROCHEMISTRY -CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (SHORT ANSWER QUESTIONS (2 MARKS EACH))
- Mention the reactions occurring at (i) anode (ii) cathode, during work...
Text Solution
|
- Give an example of a fuel cell and write the cathode and anode reactio...
Text Solution
|
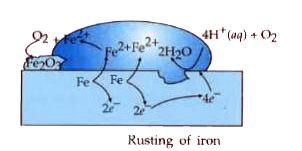
- The chemistry of corrosion of iron is essentially an electrochemical p...
Text Solution
|
- Given that the standard electrode potentials (E^(@)) of metals are: ...
Text Solution
|
- Two half-reactions of an electrochemical cell are given below : MnO...
Text Solution
|
- What type of cell is a lead storage battery ? Write the anode and the ...
Text Solution
|
- Define the terms given below : (a) Conductivity (b) Molar conductiv...
Text Solution
|
- Describe the construction of a H(2)-O(2) , fuel cell and the reactions...
Text Solution
|
- The following curve is obtained when molar conductivity Lambda(m) is p...
Text Solution
|
- The molar conductivity (Lambda(m)) of KCl solutions at different conce...
Text Solution
|
- (i) For a weak electrolyte molar conductance in dilute solution increa...
Text Solution
|
- Write the chemical equations for all the steps involved in the rusting...
Text Solution
|
- Value of standard electrode potential for the oxidation of Cl^(-) ions...
Text Solution
|
- Aqueous copper sulphate solution and aqueous silver nitrate solution a...
Text Solution
|
- How long a current of 3 amperes has to be passed through a solution of...
Text Solution
|
- Consider the following diagram in which an electrochemical cell is cou...
Text Solution
|
- What advantages do the fuel cells have over primary and secondary batt...
Text Solution
|
- Write the Nernst equation for the cell reaction in the Daniel cell. Ho...
Text Solution
|
- Consider a cell given below: Cu | Cu^(2+) || Cl^(-) | Cl(2),Pt Wri...
Text Solution
|
- Write the cell reaction of a lead storage battery when it is discharge...
Text Solution
|