Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (LONG ANSWER QUESTIONS-II (5 MARKS EACH))|16 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION A) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)|7 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (SHORT ANSWER QUESTIONS (2 MARKS EACH))|47 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|7 VideosEXAMINATION PAPER 2020
U-LIKE SERIES|Exercise SECTION D|15 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-ELECTROCHEMISTRY -CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (LONG ANSWER QUESTIONS-I (3 MARKS EACH))
- Calcualte emf of the following at 25^(@) C Fe | Fe^(2+) (0.001) || H...
Text Solution
|
- Calculate the emf of the following cell at 25^(@) C Zn| Zn^(2+) (0.0...
Text Solution
|
- Conducitivity of 2.5 xx 10^(-4) M methonic acid is 5.25 xx 10^(-5) S c...
Text Solution
|
- (a) Calculate Delta(r)G^(@) for the reaction Mg(s) + Cu^(2+) (aq) to...
Text Solution
|
- State Kohlrausch law of independent migration of ions. Why does the co...
Text Solution
|
- Calcualte the emf of the following cell at 25^(@) C Ag(s) | Ag^(+) (...
Text Solution
|
- The electrical resistance of a column of 0.05 M NaOH solution of diame...
Text Solution
|
- Calculate the equilibrium constant K for the reaction at 298 K Zn(s)...
Text Solution
|
- For the cell Zn(s) | Zn^(2+)(2 M) ||Cu^(2+) (0.5 M) | Cu(s) (a) Wr...
Text Solution
|
- (a) Calculate the charge in coulombs required for oxidation of 2 moles...
Text Solution
|
- What is nickel-cadmium cell ? State its one merit and one demerit over...
Text Solution
|
- Give reason (a) Why does an alkaline medium inhibit the rusting of i...
Text Solution
|
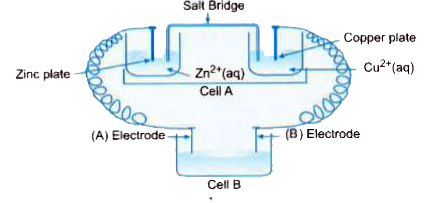
- Consider the figure below and answer the following questions : (i...
Text Solution
|
- The following chemical reaction is occurring in an electrochemical cel...
Text Solution
|
- (a) A current of 1.50 amp was passed through an electrolytic cell cont...
Text Solution
|
- Calculate the emf and DeltaG of cell reaction for the following cell ...
Text Solution
|
- Calculate the standard cell potential of the galvanic cell in which th...
Text Solution
|
- a) Calculate the charge in coulombs required for the oxidation of 2 mo...
Text Solution
|
- Conductivity of 0.00241 M acetic acid is 7.896 xx 10^(-5) S cm^(-1). C...
Text Solution
|
- (i) Solutions of two electrolytes 'A' and 'B' are diluted. The limitin...
Text Solution
|