Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION A) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)|7 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION B)|1 VideosELECTROCHEMISTRY
U-LIKE SERIES|Exercise CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (LONG ANSWER QUESTIONS-I (3 MARKS EACH))|27 VideosCOORDINATION COMPOUNDS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST|7 VideosEXAMINATION PAPER 2020
U-LIKE SERIES|Exercise SECTION D|15 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-ELECTROCHEMISTRY -CASE BASED/SOURCE-BASED INTEGRATED QUESTIONS (LONG ANSWER QUESTIONS-II (5 MARKS EACH))
- For the reaction: 2AgCl(s) + H(2)(g) (1 atm) to 2Ag (s) + 2H^(+) (0....
Text Solution
|
- (a) Write the cell reaction and calculate the emf of the following cel...
Text Solution
|
- (a) Calcualte E("cell")^(@) for the following reaction at 298 K 2Al(...
Text Solution
|
- (a) The conductivity of 0.001 mol L^(-1) solution of CH3COOH is 3.9...
Text Solution
|
- (a) The conductivity of 0.001 mol L^(-1) solution of CH3COOH is 3.905...
Text Solution
|
- (a) Calculate DeltaG^(@) and log K( c) for the following reaction at...
Text Solution
|
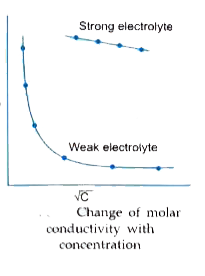
- (a) Define molar conductivity of a solution and explain how molar cond...
Text Solution
|
- What type of a battery is the lead storage battery 7 Write the anode a...
Text Solution
|
- (i) What type of a battery is lead storage battery ? Write the anode a...
Text Solution
|
- (a) State Kohlrausch law of independent migration of ions. Write an ex...
Text Solution
|
- (a) Two electrolytic cells containing silver nitrate solution and dilu...
Text Solution
|
- Define molar conductivity of a substance and describe how for weak and...
Text Solution
|
- (i) The resistance of a conductivity cell containing 0.001 M KCl solut...
Text Solution
|
- Consider the figure given alongside and answer the questions (i) to (v...
Text Solution
|
- (a) Calculate the equilibrium constant for the reaction : Cd^(2+) (a...
Text Solution
|
- (i) State the relationship amongst cell constant of a cell, resistance...
Text Solution
|