Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise LONG ANSWER QUESTION-I|56 VideosTHE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise LONG ANSWER QUESTION-II|68 VideosTHE P-BLOCK ELEMENTS
U-LIKE SERIES|Exercise VERY SHORT ANSWER QUESTIONS|75 VideosTHE D-AND F-BLOCK ELEMENTS
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION A ) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)|7 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-THE P-BLOCK ELEMENTS -SHORT ANSWER QUESTIONS
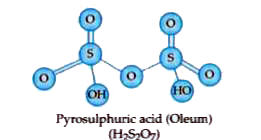
- Write the structure of the following H2S2O7
Text Solution
|
- Write the structure of the following XeO3
Text Solution
|
- Write the structure of the following N2O5
Text Solution
|
- Write the structure of the following Br3
Text Solution
|
- Write the structure of the following molecules : H2SO3
Text Solution
|
- Write the structure of the following molecules : XeOF4
Text Solution
|
- Complete the following reaction : P4 + H2O to
Text Solution
|
- Complete the following reaction : XeF4 + O2F2 to
Text Solution
|
- What happens when (i) PCl5 is heated ? (ii) H3PO3 is heated ? Writ...
Text Solution
|
- Explain the following giving an appropriate reason in case : O2 an...
Text Solution
|
- Explain the following giving an appropriate reason in case : Struct...
Text Solution
|
- State reasons for the following: The N - O bond in NO2^(-) is shor...
Text Solution
|
- State reasons for the following: SF6 is kinetically an inert subst...
Text Solution
|
- State reasons for each of the following: All the P-Cl bonds in PCl5...
Text Solution
|
- State reasons for each of the following: Sulphur has greater tenden...
Text Solution
|
- Draw the structure of phosphinic acid (H3PO2).
Text Solution
|
- Write a chemical reaction for its use as reducing agent.
Text Solution
|
- Suggest a quantitative method for estimation of the gas which protects...
Text Solution
|
- Nitrogen oxides emitted from the exhaust system of supersonic jet aero...
Text Solution
|
- Complete the following reaction equations : XeF2 (s) + H2O (l) to
Text Solution
|