Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL CALCULATION
NCERT GUJARATI|Exercise PROBLEM|4 VideosCHEMICAL CALCULATION
NCERT GUJARATI|Exercise EXAMPLE|3 VideosCHEMICAL BONDING
NCERT GUJARATI|Exercise Questions(E. Explain briefly on the following)|1 VideosCHEMICAL EQUILIBRIUM - I
NCERT GUJARATI|Exercise Questions(E. Explain briefly on the following)|2 Videos
Similar Questions
Explore conceptually related problems
NCERT GUJARATI-CHEMICAL CALCULATION -Question ( Answer the following )
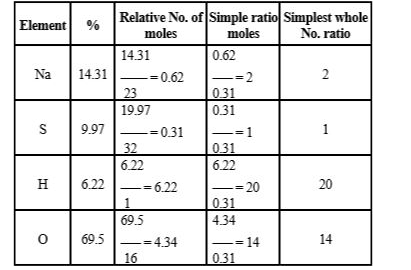
- A compound on analysis gave the following percentage composition: Na=1...
Text Solution
|
- Can two different compounds have same molecular formula ? Illustrate y...
Text Solution
|
- What are the essentials of a chemical equation ?
Text Solution
|
- What are the informations conveyed by a chemical equation ?
Text Solution
|
- Balance the following equations Fe + H2O to Fe3O4 + H2
Text Solution
|
- Balance the following equations Fe2(SO4)3 + NH3 + H2O to Fe(OH)3 + ...
Text Solution
|
- Balance the following equations KMnO4 + H2SO4 to K2SO4 + MnSO4 + H2...
Text Solution
|
- Balance the following equations K2Cr2O7+ H2SO4 to K2SO4 to K2SO4 + ...
Text Solution
|