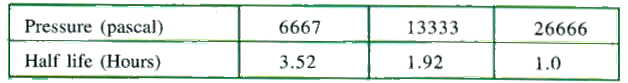Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL KINETCS-EXERCISE - 3.2
- What is a pesudo - order reaction ? Why is it so called ?
Text Solution
|
- If a second order reaction is 75% complete in 1 hr , calculate th...
Text Solution
|
- For a reaction , the reactant concentration decreases 20% in 1 hr a...
Text Solution
|
- The rate of a first order reaction is 0.04 mol L^(-1) s^(-1) after ...
Text Solution
|
- A reaction proceeds 5 times more at 60^(@)C, as it does at 30^(@)C ...
Text Solution
|
- Rate constant of a reaction is 10^(-3) s^(-1). Calculate the perc...
Text Solution
|
- For a reaction A + 2B to 2C, the kinetic data is given below: ...
Text Solution
|
- Time required for 50% completion of the first order reaction in ...
Text Solution
|
- A first order reaction is 50% complete in min at 27^(@)C ?and 10 m...
Text Solution
|
- The concentrations of N(2)O(5) decomposing in first order kinetics a...
Text Solution
|
- CH(3)O CH(3) (g) to CH(4)(g) + H(2)(g) + CO(g) . The decomposition fo...
Text Solution
|
- For the catalytic decomposition of a substance , half - lives are g...
Text Solution
|
- A drop of solution (0.05 mL) contains 2 xx 10^(6) mol of H^(+). How ...
Text Solution
|
- Half - life period of ""^(14)C is 5770 years . If and old wooden t...
Text Solution
|
- Ten gram atoms of an alpha active element disintegrated in a sealed...
Text Solution
|
- During nuclear explosion , one of the products is ""^(90)Sr with h...
Text Solution
|
- The half - life for radioactive decay of ""^(14)C is 5730 years . An...
Text Solution
|
- Acid catalysed hydrolysis of ethylacetate can be considered as an ex...
Text Solution
|
- Rate of decomposition of a substance increases by a factor 2.25 fo...
Text Solution
|
- For a first order reaction , show that time required for 99% com...
Text Solution
|