A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Lecture Sheet- Exercise-II|25 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Lecture Sheet Exercise-III|23 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise Objective Exercise - 4 (Assertion (A) & Reason (R) Type Questions)|44 VideosCHEMISTRY IN EVERY DAY LIFE
AAKASH SERIES|Exercise PRACTICE EXERCISE|29 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYNAMICS-Additional Practice Exercise
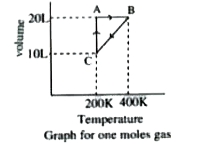
- Answer the following questions based on the diagram below involving 1 ...
Text Solution
|
- Which among the following is not an exact differential?
Text Solution
|
- A system consisting of one mole of an ideal diatomic gas absorbs 200J ...
Text Solution
|
- A mono atomic ideal gas undergoes a process in which the ratio of P to...
Text Solution
|
- 1 mole each of CaC(2), Mg(2)C(3) reacts with excess water in separate ...
Text Solution
|
- The temperature of 5 moles of a gas is decreased by 2K at constant pre...
Text Solution
|
- When an ideal gas at a pressure P, temperature T and volume V is isoth...
Text Solution
|
- Which of the following statement is incorrect?
Text Solution
|
- A gas absorbs 100 calories of heat energy and is compressed from 10L t...
Text Solution
|
- The enthalpy of neutralization of weak monoprotic acid, HA in 1M solut...
Text Solution
|
- The heat of combustion of hydrocarbon C(x)H(y) is ''a'' calories and h...
Text Solution
|
- The standard enthalpy of formation of hypothetical MgCl is -125kJ mol^...
Text Solution
|
- Calculate the resonance energy of N(2)O from the following data Delta ...
Text Solution
|
- The maximum entropy of mixing ocuurs when hexane and heptane are mixed...
Text Solution
|
- When 3.0 mole of an ideal diatomic gas is heated and compressed simult...
Text Solution
|
- underset(P(1), V(1), T(1))("State") A overset("Rev")rarr underset(P(2)...
Text Solution
|
- During winters, moisture condenses in the form of dew and can be seen ...
Text Solution
|
- Assuming Delta H^(0) and Delta S^(0) do not change with temperature th...
Text Solution
|
- For the reaction X(2)O(4(l)) rarr 2XO(2(g)), Delta U= 2.1 "kcal", Delt...
Text Solution
|
- The correct signs of Delta S for the following four processes respecti...
Text Solution
|
- Assume that C(6)H(6) and C(6)H(5)CH(3) form an ideal solution then Del...
Text Solution
|