A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
U-LIKE SERIES|Exercise FILL IN THE BLANKS.|13 VideosATOMS
U-LIKE SERIES|Exercise TRUE OR FALSE .|5 VideosATOMS
U-LIKE SERIES|Exercise CASE BASED/SOURCE - BASED INTERGRATED QUESTIONS|10 VideosALTERNATING CURRENT
U-LIKE SERIES|Exercise SELF ASSESSMENT TEST (SECTION -C)|2 VideosCBSE EXAMINATION PAPER 2020
U-LIKE SERIES|Exercise SECTION D|12 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-ATOMS-MULTIPLE CHOICE QUESTIONS
- In hydrogen atom which quantity is integral multiple of
Text Solution
|
- The energy of electron in first excited state of H-atom is - 3.4 eV. I...
Text Solution
|
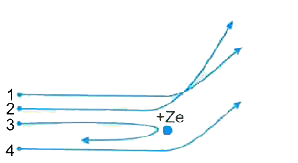
- The diagram 12.03 shows the path of four a-particles of the same energ...
Text Solution
|
- Bohr's atom model presumes that.
Text Solution
|
- In the nth stable orbit of a hydrogen atom, the energy of an electron ...
Text Solution
|
- The Lyman series of hydrogen spectrum lies in which of the following r...
Text Solution
|
- Size of an atom is of the order of
Text Solution
|
- Hydrogen atoms in the ground state (E = - 13.6 eV) are excited by mono...
Text Solution
|
- The ratio of the energies of the hydrogen atom in its first excited st...
Text Solution
|
- Energy levels A, B and C of a certain atom correspond to increasing va...
Text Solution
|
- Consider an electron in the nth orbit of a hydrogen atom in the Bohr's...
Text Solution
|
- Ratio of the wavelengths of line of Lyman series and first line of Bal...
Text Solution
|
- In Rutherford's scattering experiment if impact parameter is zero then...
Text Solution
|
- As per Bohr atom model if the radius of the first orbit in an hydrogen...
Text Solution
|
- The concept of stationary (non-radiating) orbits was proposed by
Text Solution
|
- The first line of the Paschen series in hydrogen spectrum has a wavele...
Text Solution
|
- Whenever a hydrogen atom emits a photon in the Balmer series, it
Text Solution
|
- The diagram shows the energy level for an electron in a hydrogen atom....
Text Solution
|
- The energy of the highest energy photon of Balmer series of hydrogen a...
Text Solution
|
- Energy of an electron in an excited state of hydrogen atom is - 3.4 eV...
Text Solution
|