Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL REACTIONS AND EQUATIONS
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS|21 VideosCHEMICAL REACTIONS AND EQUATIONS
U-LIKE SERIES|Exercise VERY SHORT ANSWER QUESTIONS|31 VideosCBSE EXAMINATION PAPER 2020 (SOLVED)
U-LIKE SERIES|Exercise SECTION C|9 VideosMETALS AND NON METALS
U-LIKE SERIES|Exercise LONG ANSWER QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
U-LIKE SERIES-CHEMICAL REACTIONS AND EQUATIONS -SHORT ANSWER QUESTIONS
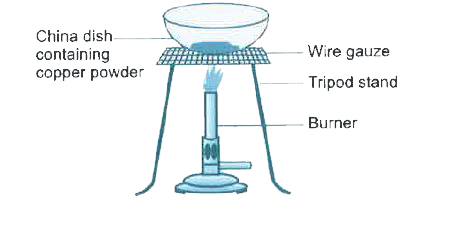
- Look at the figure given below and answer the following questions ...
Text Solution
|
- Look at the figure given below and answer the following questions ...
Text Solution
|
- Look at the figure given below and answer the following questions ...
Text Solution
|
- Balance the following chemical equations : Mg+N(2)toMg(3)N(2)
Text Solution
|
- Balance the following chemical equations : Al+Cl(2)toAlCl(3)
Text Solution
|
- Balance the following chemical equations : Pb(NO(3))(2)overset("Heat...
Text Solution
|
- Take 3 g of barium hydroxide in a test tube, now add about 2 g ammoniu...
Text Solution
|
- Take 3 g of barium hydroxide in a test tube, now add about 2 g ammoniu...
Text Solution
|
- Take 3 g of barium hydroxide in a test tube, now add about 2 g ammoniu...
Text Solution
|
- Write the balanced chemical equations for the following reactions : ...
Text Solution
|
- Write the balanced chemical equations for the following reactions : ...
Text Solution
|
- Write the balanced chemical equations for the following reactions : ...
Text Solution
|
- Classify the following chemical reactions as exothermic or endothermic...
Text Solution
|
- Classify the following chemical reactions as exothermic or endothermic...
Text Solution
|
- Classify the following chemical reactions as exothermic or endothermic...
Text Solution
|
- Classify the following chemical reactions as exothermic or endothermic...
Text Solution
|
- Classify the following chemical reactions as exothermic or endothermic...
Text Solution
|
- Classify the following chemical reactions as exothermic or endothermic...
Text Solution
|
- Write chemical equations for the reactions taking place when : magne...
Text Solution
|
- Write chemical equations for the reactions taking place when : sodiu...
Text Solution
|