Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (VERY SHORT ANSWER TYPE QUESTIONS)|18 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (SHORT ANSWER TYPE QUESTION-I)|15 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 1 (SHORT ANSWER TYPE QUESTIONS)|10 VideosELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic - 3 ELECTROLYSIS , LAWS OF ELECTROLYSIS, BATTERIES, FUEL CELLS AND CORROSION (LONG ANSWER TYPE QUESTIONS)|4 VideosHALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic 2 (Properties of Haloarenes and Haloalkanes) (Long Answer Type Questions - II)|1 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS -TOPIC 1 (LONG ANSWER TYPE QUESTIONS-I)
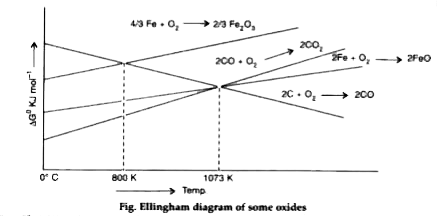
- On the basis of Ellingham's diagram explain the principle of extractio...
Text Solution
|
- Explain with equation Van-Arkel method for refining of zircnium.
Text Solution
|
- Describe parke's process of desilverisation of lead.
Text Solution
|
- Outline the principles of refining metals by the following methods : ...
Text Solution
|
- Differentiate between 'minerals' and 'ores'.
Text Solution
|
- Explain hydraulic washing used for ore concentration.
Text Solution
|
- How is cast iron different form pig iron?
Text Solution
|