Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (LONG ANSWER TYPE QUESTIONS-I)|13 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (LONG ANSWER TYPE QUESTIONS-II)|2 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (VERY SHORT ANSWER TYPE QUESTIONS)|18 VideosELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic - 3 ELECTROLYSIS , LAWS OF ELECTROLYSIS, BATTERIES, FUEL CELLS AND CORROSION (LONG ANSWER TYPE QUESTIONS)|4 VideosHALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic 2 (Properties of Haloarenes and Haloalkanes) (Long Answer Type Questions - II)|1 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS -TOPIC 2 (SHORT ANSWER TYPE QUESTION-I)
- Suggest any two methods to prevent corrosion of iron.
Text Solution
|
- What is the role of lime stone and coke in the extraction of iron usin...
Text Solution
|
- Give reasons : (i) Copper displaces silver from silver nitrate solut...
Text Solution
|
- Give reason : Copper is more electro positive than silver.
Text Solution
|
- Copper is a stronger reducing agent than silver.
Text Solution
|
- SRP of copper is less than that for silver.
Text Solution
|
- Iron pipe are usually coated with zinc.
Text Solution
|
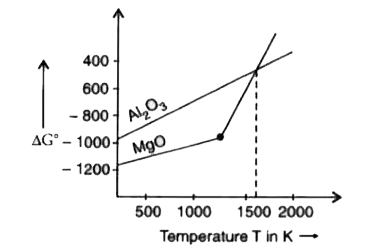
- With the help of Ellingham diagram, explain why Al can reduce MgO at h...
Text Solution
|
- Why copper matte is put in silica lined converter?
Text Solution
|
- What is the role of cryolite in the metallurgy of aluminium?
Text Solution
|
- Give any three uses of copper.
Text Solution
|
- Give any two uses of zinc.
Text Solution
|
- How is wrought iron prepared from cast iron?
Text Solution
|
- Give any two uses of iron.
Text Solution
|
- Explain the extraction of zinc from zinc oxide or from zinc blende (Zn...
Text Solution
|