Text Solution
Verified by Experts
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (LONG ANSWER TYPE QUESTIONS-II)|2 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (SHORT ANSWER TYPE QUESTION-I)|15 VideosELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic - 3 ELECTROLYSIS , LAWS OF ELECTROLYSIS, BATTERIES, FUEL CELLS AND CORROSION (LONG ANSWER TYPE QUESTIONS)|4 VideosHALOALKANES & HALOARENES
OSWAAL PUBLICATION|Exercise Topic 2 (Properties of Haloarenes and Haloalkanes) (Long Answer Type Questions - II)|1 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS -TOPIC 2 (LONG ANSWER TYPE QUESTIONS-I)
- How copper is refined by electrolytic method?
Text Solution
|
- How is pure alumina obtained from bauxite by leaching process.
Text Solution
|
- Describe the three steps involved in the leaching of bauxite to get pu...
Text Solution
|
- In the extraction of Aluminium by Hall- Herault process: Give the e...
Text Solution
|
- (a) Name the reducing agent used in the extraction of zinc oxide. Give...
Text Solution
|
- Draw labelled diagram of Hall-Heroult electrolytic cell for the extrac...
Text Solution
|
- (i) Draw a neat labelled diagram of blast furnace used for the extract...
Text Solution
|
- (a) Write chemical reactions taking place in the blast furnace at redu...
Text Solution
|
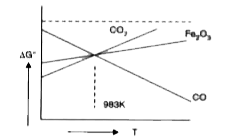
- Draw Ellingham diagram [DeltaG^(@)VsT] for the oxidation of carbon mon...
Text Solution
|
- Draw a neat diagram of blast furnance and describe and describe the re...
Text Solution
|
- State the role of silica in the metallurgy of Copper.
Text Solution
|
- The value of Delta(f)G^(@) for formation Cr(2)O(3) is -540 kJ mol^(-1)...
Text Solution
|
- The choice of a reducing agent in a particular case depends on thermod...
Text Solution
|