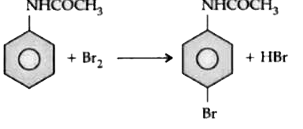
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Long Answer Type Questions-I)|2 VideosII PUC TOPPER'S ANSWER MARCH - 2017
OSWAAL PUBLICATION|Exercise Part - D|11 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 4 (GROUP - 18 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - II)|3 Videos
Similar Questions
Explore conceptually related problems