Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic -2 REDOX REACTION, ELECTROCHEMICAL CELL, GALVANIC CELL, EMF OF CELL, STANDARD ELECTRODE POTENTIAL, NERNST EQUATION (LONG ANSWER TYPE QUESTION -II)|8 VideosELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic - 3 ELECTROLYSIS , LAWS OF ELECTROLYSIS, BATTERIES, FUEL CELLS AND CORROSION (VERY SHORT ANSWER TYPE QUESTIONS)|6 VideosELECTRO-CHEMISTRY
OSWAAL PUBLICATION|Exercise Topic -2 REDOX REACTION, ELECTROCHEMICAL CELL, GALVANIC CELL, EMF OF CELL, STANDARD ELECTRODE POTENTIAL, NERNST EQUATION (SHORT ANSWER TYPE QUESTIONS)|19 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(LONG ANSWER TYPES QUESTIONS)|6 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC 2 (LONG ANSWER TYPE QUESTIONS-II)|2 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-ELECTRO-CHEMISTRY -Topic -2 REDOX REACTION, ELECTROCHEMICAL CELL, GALVANIC CELL, EMF OF CELL, STANDARD ELECTRODE POTENTIAL, NERNST EQUATION (LONG ANSWER TYPE QUESTION -I)
- Calculate the e.m.f. of the cell in which the following reaction takes...
Text Solution
|
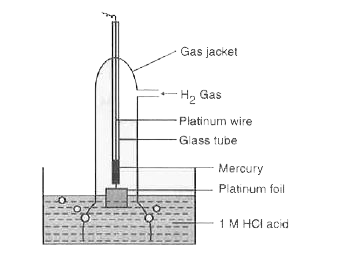
- Describe the construction and working of standard hydrogen electrode.
Text Solution
|
- Explain construction of Daniel cell. Write half cell reactions. How is...
Text Solution
|
- Calculate the emf of the following cell at 298K, Fe(s)Fe^(2+)(0.001...
Text Solution
|
- The cell in which of the following reaction occurs: 2Fe^(3+)(aq)+2I^...
Text Solution
|
- A strip of nickel metal is placed in a 1molar solution of Ni(NO3)2 and...
Text Solution
|
- Write the Nernst equation and compute the emf of the following cell at...
Text Solution
|