Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
OSWAAL PUBLICATION|Exercise Topic - 1(RATE OF CHEMICAL REACTION AND FACTORS AFFECTING RATE OF REACTION ) (Short Answer Type )Questions|9 VideosCHEMICAL KINETICS
OSWAAL PUBLICATION|Exercise Topic - 1(RATE OF CHEMICAL REACTION AND FACTORS AFFECTING RATE OF REACTION ) (Long Answer Type Questions - I)|3 VideosBIOMOLECULES
OSWAAL PUBLICATION|Exercise TOPIC -II (LONG ANSWER TYPE QUESTIONS)|2 VideosCHEMISTY IN EVERYDAY LIFE
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS-I|1 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CHEMICAL KINETICS-Topic - 2 (ORDER OF A REACTION , INTEGRATED RATE EQUATIONS AND HALF LIFE OF A REACTION )Long Answer Type Questions - II)
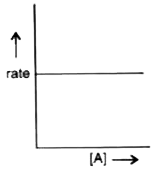
- For a reaction the graph of the rate of the reaction against molar con...
Text Solution
|
- The half-life period of a certain reaction is directly proportional to...
Text Solution
|
- Define half life period for a reaction and how it is related to the or...
Text Solution
|
- a) The rate of a particular reaction doubles when the temperature chan...
Text Solution
|
- b) Show that the half - life period of a first order reaction is indep...
Text Solution
|