Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-II PUC MARCH - 2017-PART - C
- In the extraction of Aluminium by Hall- Herault process: Give the e...
Text Solution
|
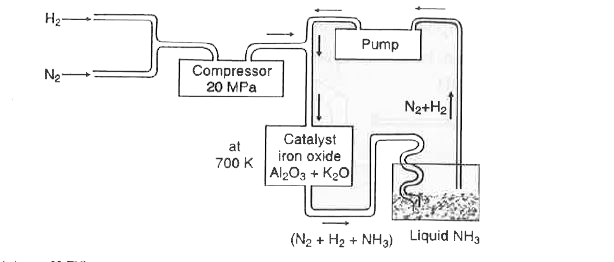
- In the manufactore of ammonia by Haber's process, write the flow chart...
Text Solution
|
- (i) Mention any two reasons for the anomalous behaviour of oxygen. (...
Text Solution
|
- Complete the following equations. a) underset("(cold and dil)")(2NaO...
Text Solution
|
- Which element of 3d series exhibits maximum oxidation state?
Text Solution
|
- How is KMnO(4) [Potassium permanganate] is prepared from MnO(2)? Write...
Text Solution
|
- With the help of Valence Bond theory account for hybridisation, geomet...
Text Solution
|
- Write the cis and trans isomeric structures of [Fe(NH(3))(2)(CN)(4)]^(...
Text Solution
|