Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-II PUC MARCH - 2017-PART - D
- An element having atomic mass 63.1 g/mol has face centered cubic unit ...
Text Solution
|
- (a) 10 g of non-electrolyte solute dissolved in 50 g of benzene lowere...
Text Solution
|
- (a) The electrode potential for the Daniell cell given below is 1.1 V....
Text Solution
|
- Derive an integrated rate equation for rate constant of a zero order r...
Text Solution
|
- (a) Give any two differences between lyophilic and lyophobic colloids....
Text Solution
|
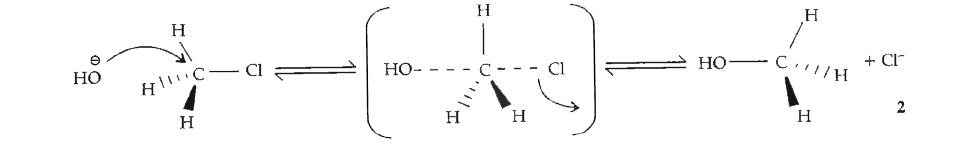
- Write SN^(2) mechanism of the conversion of methyl chloride to methyl ...
Text Solution
|
- Write the mechanism of acid catalysed dehydration of ethanol to ethene...
Text Solution
|
- What is the effect of electron withdrawing group on the acidity of car...
Text Solution
|
- i) Write IUPAC name of CH(3)CH(2)NH(2). ii) Arrange the following am...
Text Solution
|
- Give an example for i) Globular proteins. ii) Naturally occurring ...
Text Solution
|
- Name the monomer present in the following polymer i) Poly vinyl chlo...
Text Solution
|