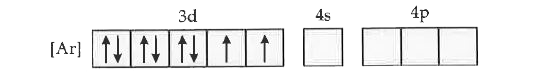
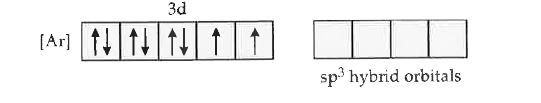
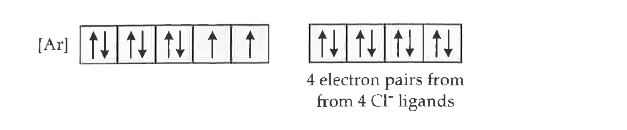
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-II PUC MARCH - 2018-PART - C
- Explain the process of obtaining "blister copper" from "copper matte" ...
Text Solution
|
- Write the equation involved in the manufacture of nitric acid by Ostwa...
Text Solution
|
- (a) How is ozonised oxygen prepared in the laboratory? Give equation. ...
Text Solution
|
- Complete the following equations : a) 2NaOH+Cl(2)rarrNaCl+"……"+H(2)O...
Text Solution
|
- How is potassium permanganate ( KMnO4) prepared from MnO2 ? write the ...
Text Solution
|
- Why 3d-series of elements acts as good catalyst?
Text Solution
|
- Explain the hybridisation, geometry and magnetic property of [Ni(Cl)(4...
Text Solution
|
- Write the IUPAC name of : [Co(NH(3))(4)(H(2)O)Cl]Cl(2)
Text Solution
|