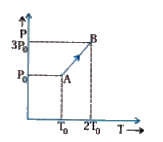
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYANMICS
KUMAR PRAKASHAN|Exercise Section-F Questions from Module|20 VideosTHERMODYANMICS
KUMAR PRAKASHAN|Exercise Question Paper|11 VideosTHERMODYANMICS
KUMAR PRAKASHAN|Exercise Section-D Ncert Exemplar Solution (Long Answer )|5 VideosTHERMAL PROPERTIES OF MATTER
KUMAR PRAKASHAN|Exercise Question Paper (Section - D) (Answer following in brief :) Each carry 4 marks|1 VideosUNITS AND MEASUREMENT
KUMAR PRAKASHAN|Exercise Section -F (Questions from Module )|20 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-THERMODYANMICS -Section-E MCQs
- The relation between pressure and temperature of a monoatomic gas in a...
Text Solution
|
- n moles of a monoatomic gas is carried round the reversible rectangula...
Text Solution
|
- Pressure versus temperature graph of an ideal gas is as shown in figur...
Text Solution
|
- Graph of specific heat at constant volume for a monoatomic gas is
Text Solution
|
- For an adiabatic process
Text Solution
|
- For cyclic process which of the following quantity is zero ?
Text Solution
|
- 1 "cm"^3 of water at 1 atm pressure is converted into steam, volume of...
Text Solution
|
- Two rigid boxes containing different ideal gases are placed on a table...
Text Solution
|
- A carnot engine having an efficiency of eta=1/10 as heat engine is use...
Text Solution
|
- Helium gas goes through a cycle ABCDA as shown in figure. Efficiency o...
Text Solution
|
- One mole of diatomic ideal gas undergoes a cyclic process ABC as shown...
Text Solution
|
- Figure below shows two paths that may be taken by a gas to go from a s...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- The coefficient of performance of a refrigerator is 5. If the temperat...
Text Solution
|
- An ideal gas is compressed to half its initial volume by means of seve...
Text Solution
|
- The value of coefficient of volume expansion of glycerin is 5 xx 10^(-...
Text Solution
|
- 4.0 g of a gas occupies 22.4 litres at NTP. The specific heat capacity...
Text Solution
|
- A gas is compressed isothermally to half its initial volume. The same ...
Text Solution
|
- A refrigerator works between 4^@C and 30^@C. It is required to remove ...
Text Solution
|
- Two identical bodies are made of a material for which the heat capacit...
Text Solution
|