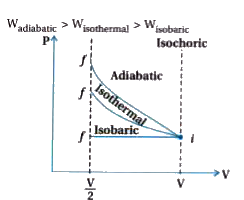
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYANMICS
KUMAR PRAKASHAN|Exercise Section-F Questions from Module|20 VideosTHERMODYANMICS
KUMAR PRAKASHAN|Exercise Question Paper|11 VideosTHERMODYANMICS
KUMAR PRAKASHAN|Exercise Section-D Ncert Exemplar Solution (Long Answer )|5 VideosTHERMAL PROPERTIES OF MATTER
KUMAR PRAKASHAN|Exercise Question Paper (Section - D) (Answer following in brief :) Each carry 4 marks|1 VideosUNITS AND MEASUREMENT
KUMAR PRAKASHAN|Exercise Section -F (Questions from Module )|20 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-THERMODYANMICS -Section-E MCQs
- One mole of diatomic ideal gas undergoes a cyclic process ABC as shown...
Text Solution
|
- Figure below shows two paths that may be taken by a gas to go from a s...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- The coefficient of performance of a refrigerator is 5. If the temperat...
Text Solution
|
- An ideal gas is compressed to half its initial volume by means of seve...
Text Solution
|
- The value of coefficient of volume expansion of glycerin is 5 xx 10^(-...
Text Solution
|
- 4.0 g of a gas occupies 22.4 litres at NTP. The specific heat capacity...
Text Solution
|
- A gas is compressed isothermally to half its initial volume. The same ...
Text Solution
|
- A refrigerator works between 4^@C and 30^@C. It is required to remove ...
Text Solution
|
- Two identical bodies are made of a material for which the heat capacit...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a process described by t...
Text Solution
|
- The temperature inside a refrigerator is t2^@C and the room temperatur...
Text Solution
|
- The volume of one mole of an ideal gas with adiabatic exponent gamma ...
Text Solution
|
- One mole of gas obey the equation of state P(V - b) = RT and changes i...
Text Solution
|
- Thermodynamic process are indicated in the following diagram. Then mat...
Text Solution
|
- A sample of 0.1 g of water at 100^@ C and normal pressure (1.013 xx 10...
Text Solution
|
- Two wires are made of the same material and have the same volume. The ...
Text Solution
|
- The volume (V) of a monoatomic gas varies with its temperature (T), as...
Text Solution
|
- The efficiency of an ideal heat engine working between the freezing po...
Text Solution
|
- Two moles of an ideal monoatomic gas occupies a volume V at 27^@ C. Th...
Text Solution
|