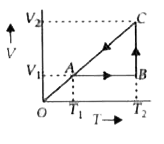
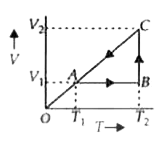
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
MTG GUIDE|Exercise NEET (ITALS)|25 VideosTHERMODYNAMICS
MTG GUIDE|Exercise AIPMT/NEET (MCQs)|26 VideosTHERMODYNAMICS
MTG GUIDE|Exercise AIPMT/NEET (MCQs)|26 VideosPROPERTIES OF BULK MATTER
MTG GUIDE|Exercise AIPMT/NEET (MCQs)|40 VideosWORK, ENERGY AND POWER
MTG GUIDE|Exercise AIPMT/NEET (MCQs)|34 Videos
Similar Questions
Explore conceptually related problems
MTG GUIDE-THERMODYNAMICS-NEET Cafe (Topicwise practice Questions)
- An ideal gas with pressure P, volume V and temperature T is expanded i...
Text Solution
|
- A given mass of gas is compressed isothermally untal its pressure is d...
Text Solution
|
- A cyclic process for 1 mole of an ideal gas is shown in the V-T diagra...
Text Solution
|
- Starting with the same initial conditions, an ideal gas expands from v...
Text Solution
|
- An ideal gas is taken round a cyclic process represened by the triangl...
Text Solution
|
- 0.2 moles of an ideal gas is taken round the cycle ABC as shown in the...
Text Solution
|
- A thermodynamic process is as shown in figure such that P(A) = 3 xx ...
Text Solution
|
- The thermodynamic process in which no work is done on or by the gas is
Text Solution
|
- The work done by a gas is maximum when it expands
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown in the P-V diagra...
Text Solution
|
- No heat flows between the system and surroundings. Then the thermdynam...
Text Solution
|
- The volume of one mole of an ideal gas changes from V to 2V at tempera...
Text Solution
|
- "Heat cannot itself flow from a body at lower temperature to the body ...
Text Solution
|
- The temperature T(1) and T(2) of heat reservoirs in the ideal Carnot e...
Text Solution
|
- A Carnot engine operates with source at 500 K and sink at 375 K. Engin...
Text Solution
|
- A reversible engine converts one-sixth of the heat input into work. Wh...
Text Solution
|
- The efficiency of a carnot engine working between temperature T(1) and...
Text Solution
|
- An engineer claims to have made an engine delivering 10 kW power with ...
Text Solution
|
- A Carnot engine operates with source at 127^(@)C and sink at 27^(@)C. ...
Text Solution
|
- An ideal gas hea engine operates in a Carnot cycle between 227^(@)C an...
Text Solution
|