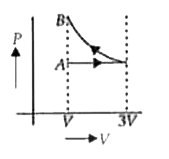
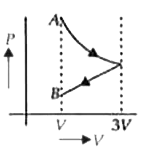
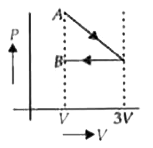
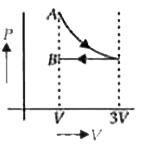
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG GUIDE-THERMODYNAMICS-AIPMT/NEET (MCQs)
- A mass of diatomic gas (gamma = 1.4) at a pressure of 2 atmosphere is ...
Text Solution
|
- A thermodynamic system is taken through the cycle ABCD as shown in fig...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
- An ideal gas goes from state A to state B via three different processe...
Text Solution
|
- In the given (V-T) diagram, what is the relation between pressures P(1...
Text Solution
|
- A gas is taken through the cycle A rarr B rarr C rarr A, as shown. Wha...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- A monoatomic gas at a pressure P, having a volume V expands isothermal...
Text Solution
|
- A thermodynamic system undergoes cyclic process ABCDA as shown in figu...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- Figure below shows two paths that may be taken by a gas to go from sta...
Text Solution
|
- An ideal gas is compressed to haf f its initial volume by means of sev...
Text Solution
|
- The coefficient of performace of a refrigerator is 5. If the temperatu...
Text Solution
|
- A gas is compressed isothermally to half its initial volume. The same ...
Text Solution
|
- A refrigerator works between 4^(@)C and 30^(@)C. It is required to rem...
Text Solution
|
- One mole of an ideal monatomic gas undergoes a process described by th...
Text Solution
|
- The temperature inside a refrigerator is t(2)^(@)C and the room temper...
Text Solution
|
- Thermodynamic process are indicated in the following diagram. Mat...
Text Solution
|
- A carnot engine having an efficiency of (1)/(10) as heat engine, is us...
Text Solution
|
- A sample of 0.1 g of water at 100^(@)C and normal pressure (1.013 xx 1...
Text Solution
|