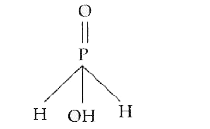
Text Solution
Verified by Experts
Topper's Solved these Questions
P - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 1 (GROUP - 15 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(SHORT ANSWER TYPE QUESTIONS)|24 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 1 (GROUP - 15 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - I)|13 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Long Answer Type Questions-II )|3 VideosPOLYMERS
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS - II|2 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-P - BLOCK ELEMENTS-TOPIC - 4 (GROUP - 18 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - II)
- What is the basicity of H(3)PO(2) acid and why?
Text Solution
|
- Write the structures of XeO(3), XeF(6), XeF(4), XeOF(4), XeF(2)
Text Solution
|
- (i) Write the balanced reaction for obtaining XeO(3) and XeOF(4) from ...
Text Solution
|
- Complete the following chemical reaction equations : (i) P(4)+SO(2)C...
Text Solution
|