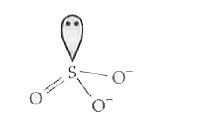
Text Solution
Verified by Experts
Topper's Solved these Questions
P - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (GROUP - 16 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(SHORT ANSWER TYPE QUESTIONS)|14 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (GROUP - 16 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - I)|18 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 1 (GROUP - 15 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - I)|13 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Long Answer Type Questions-II )|3 VideosPOLYMERS
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS - II|2 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-P - BLOCK ELEMENTS-TOPIC - 2 (GROUP - 15 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS)
- What happens when (i) Conc. H(2)SO(4) is added to CaF(2). (ii) SO(3) i...
Text Solution
|
- Why does O(3) act as a powerful oxidizing agent?
Text Solution
|
- Draw the structure of H(2)S(2)O(8).
Text Solution
|
- Write the structure of oleum (H(2)S(2)O(7))
Text Solution
|
- Predict the shape and the asked angle (90^(@)" or more or less") in th...
Text Solution
|
- Oxygen shows catenation behaviour less than sulphur.
Text Solution
|
- Sulphur has greater tendency for catenation than oxygen why?
Text Solution
|
- Oxygen is a gas but sulphur a solid. Explain.
Text Solution
|
- Why are the two S-O bonds in SO(2) molecule of equal strength?
Text Solution
|
- Draw the structure of O(3) molecule.
Text Solution
|
- Write the valence shell electronic configuration group - 16 elements.
Text Solution
|
- How does atomic and ionic radii changes with in the group - 16 element...
Text Solution
|
- Ionisation enthalphy of group - 16 elements decreases down the group W...
Text Solution
|
- Group - 16 elements have lower ionisation enthalpy values compared to ...
Text Solution
|
- Out of O and S which has higher negative electron gain enthalpy and wh...
Text Solution
|
- How does electronegativity of group - 16 elements changes down the g r...
Text Solution
|
- Oxygen and sulphur have difference in their melting point and boiling ...
Text Solution
|
- Explain the reactivity of group - 16 elements with oxygen.
Text Solution
|
- How is oxygen prepared in the laboratory?
Text Solution
|
- Name the catalyst used in the preparation of oxygen from KClO(3) or de...
Text Solution
|