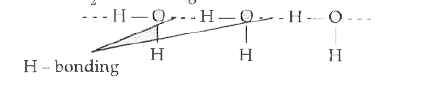
Text Solution
Verified by Experts
Topper's Solved these Questions
P - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (GROUP - 16 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS - I)|18 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 3 (GROUP - 17 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(VERY SHORT ANSWER TYPE QUESTIONS)|23 VideosP - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (GROUP - 15 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(LONG ANSWER TYPE QUESTIONS)|25 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
OSWAAL PUBLICATION|Exercise CYANIDES , ISOCYANIDES AND DIAZONIUM SALTS PREPARATION , PHYSICAL AND CHEMICAL PROPERTIES USES ( Long Answer Type Questions-II )|3 VideosPOLYMERS
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS - II|2 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-P - BLOCK ELEMENTS-TOPIC - 2 (GROUP - 16 ELEMENTS, THEIR PROPERTIES AND SOME IMPORTANT COMPOUNDS)(SHORT ANSWER TYPE QUESTIONS)
- What happens when concentrated sulphuric acid is heated with oxalic ac...
Text Solution
|
- Mention two uses of sulphuric acid.
Text Solution
|
- How does hot and concentrated sulphuric acid react with aluminium met...
Text Solution
|
- Group - 16 elements have lower ionisation enthalpy values compared to ...
Text Solution
|
- Complete the following reactions : (i) C(2)H(4)+O(2)rarr (ii) 4Al+...
Text Solution
|
- Why does O(3) act as a powerful oxidizing agent?
Text Solution
|
- Why is H(2)O a liquid and H(2)S a gas?
Text Solution
|
- What are basic oxides ? Give example.
Text Solution
|
- What are amphoteric oxides ? Give examples.
Text Solution
|
- How ozone is formed from atmospheric oxygen in the atmosphere/ What is...
Text Solution
|
- How is ozone prepared? What happens when ozone react with lead sulphid...
Text Solution
|
- Give reasons : A silent electric discharge is used in the preparation ...
Text Solution
|
- How do you estimate ozone quantitatively?
Text Solution
|
- What is an acidic oxide? Give examples.
Text Solution
|