Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-A QUESTIONS (SIMPLE OXIDES)|1 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-A QUESTIONS (SULPHUR-ALLOTROPIC FORMS)|1 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-A QUESTIONS (PHOSPHORUS HALIDES)|4 VideosTHE D-AND F-BLOCK ELMENTS
KUMAR PRAKASHAN|Exercise Section -E MCQs asked in GUJCET/Board Exams)|50 VideosTHE SOLID STATE
KUMAR PRAKASHAN|Exercise SECTION - E (MULTIPLE CHOICE QUESTIONS)(MCQs ASKED IN BOARD EXAMS)|35 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-THE P-BLOCK ELEMENTS -SECTION-A QUESTIONS (OXOACIDS OF PHOSPHORUS)
- Draw the structures of following oxoacids of phosphorus : (i) Orthop...
Text Solution
|
- Explain chemical behaviour of oxoacids of phosphorus.
Text Solution
|
- Explain occurence of group-16 elements.
Text Solution
|
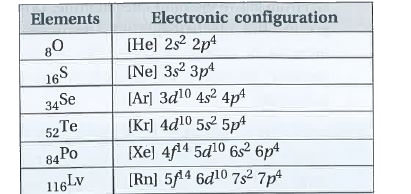
- State the electronic configurations of group-16 elements.
Text Solution
|
- Explain variations in atomic radii and ionisation enthalpies in group-...
Text Solution
|
- Explain variations in electron gain enthalpy and electronegativity of ...
Text Solution
|
- Discuss the physical properties of group-16 elements.
Text Solution
|
- Write a note on oxidation state of group-16 elements.
Text Solution
|
- Write a note on hydride compounds of Group-16 elements.
Text Solution
|
- Write a note on oxides of group-16 elements
Text Solution
|
- Write a note on oxides of group-16 elements
Text Solution
|
- Write a note on halide compounds of group-16 elements.
Text Solution
|
- Discuss the anomalous behaviour of oxygen.
Text Solution
|
- Write a preparation of dioxygen.
Text Solution
|
- Explain the physical and chemical properties of dioxygen.
Text Solution
|
- State uses of dioxygen.
Text Solution
|