Text Solution
Verified by Experts
Topper's Solved these Questions
THE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION-C TEXTUAL EXERCISE|40 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SECTION -D (NCERT EXEMPLAR SOLUTION) (MULTIPLE CHOICE QUESTIONS (MCQS))|27 VideosTHE P-BLOCK ELEMENTS
KUMAR PRAKASHAN|Exercise SELF- PRACTICE QUESTIONS (GIVE REASONS FOR THE FOLLOWING):|14 VideosTHE D-AND F-BLOCK ELMENTS
KUMAR PRAKASHAN|Exercise Section -E MCQs asked in GUJCET/Board Exams)|50 VideosTHE SOLID STATE
KUMAR PRAKASHAN|Exercise SECTION - E (MULTIPLE CHOICE QUESTIONS)(MCQs ASKED IN BOARD EXAMS)|35 Videos
KUMAR PRAKASHAN-THE P-BLOCK ELEMENTS -SECTION-B (INTEXT QUESTIONS AND ANSWERS)
- Mention the conditions required to maximise the yield of ammonia.
Text Solution
|
- How does ammonia react with a solution of Cu^(2+)?
Text Solution
|
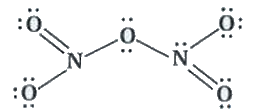
- What is the covalence of nitrogen in N(2)O(5) ?
Text Solution
|
- (a) Bond angle in PH4 is higher than that in PH3. Why ? (b) What is ...
Text Solution
|
- What happens when white phosphorus is heated with concentrated NaOH so...
Text Solution
|
- What happens when PCl5 is heated ?
Text Solution
|
- Write a balanced equation for the reaction of PCl5 with water.
Text Solution
|
- What is the basicity of H(3)PO(3) is heated?
Text Solution
|
- What happens when H3PO(3) is heated ?
Text Solution
|
- List the important sources of sulphur.
Text Solution
|
- Write the order of thermal stability of the hydrides of Group-16 eleme...
Text Solution
|
- Why is H2O a liquid and H2S a gas ?
Text Solution
|
- Which of the following does not react with oxygen directly ? Zn, Ti, P...
Text Solution
|
- Complete the following reactions : (i) C(2)H(4) + O(2) to (ii) 4Al...
Text Solution
|
- Why does O(3) act as a powerful oxidising agent ?
Text Solution
|
- How is O(3) estimated quantitatively ?
Text Solution
|
- What happens when sulphur dioxide is passed through an aqueous solutio...
Text Solution
|
- Comment on the nature of two S-0 bonds formed in SO(2) molecule. Are t...
Text Solution
|
- How is the presence of SO2 detected ?
Text Solution
|
- Mention three areas in which H2SO4 plays an important role.
Text Solution
|