Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
KUMAR PRAKASHAN|Exercise SECTION - D NCERT EXEMPLAR SOLUTION (Multiple Choice Questions)|35 VideosSOLUTIONS
KUMAR PRAKASHAN|Exercise SECTION - D NCERT EXEMPLAR SOLUTION (Short Answer Type Questions)|12 VideosSOLUTIONS
KUMAR PRAKASHAN|Exercise SECTION - B INTEX QUESTIONS AND ANSWERS|13 VideosSAMPLE QUESTION PAPER
KUMAR PRAKASHAN|Exercise [PART-B] SECTION - C|2 VideosSURFACE CHEMISTRY
KUMAR PRAKASHAN|Exercise SECTION - E (MCQs asked in GUJCET/Board Exams)|52 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-SOLUTIONS -SECTION - C TEXTUAL EXERCISE
- At 27^(@)C temperature, 36 gm glucose is in 1 litre aqueous solution h...
Text Solution
|
- Suggest the most important type of intermolecular attractive interacti...
Text Solution
|
- Based on solute - solvent interactions, arrange the following in order...
Text Solution
|
- Amongst the following compounds, identify which are insoluble, partial...
Text Solution
|
- If the density of some lake water 1.25g mol^(-1) is and contains 92 g ...
Text Solution
|
- If the solubility product of CuS is 6xx10^(-15), calculate the maximum...
Text Solution
|
- Calculate the mass percentage of aspirin (C(9)H(8)O(4)) in acetonitril...
Text Solution
|
- Nalophene (C(19)H(21)NO(3)), similar to morphine, is used to combat wi...
Text Solution
|
- Calculate the amount of benzoic acid (C(6)H(5)COOH) required for prepa...
Text Solution
|
- The depression in freezing point of water observed for the same amount...
Text Solution
|
- Calculate the depression in the freezing point of water when 10 g of C...
Text Solution
|
- 19.5 g of CH(2)FCOOH is dissolved in 500 g of water. The depression in...
Text Solution
|
- Vapour pressure of water at 293 K is 17.535 mm Hg. Calculate the vapou...
Text Solution
|
- Henry's law constant for the molality of methane in benzene at 298 K i...
Text Solution
|
- 100 g of liquid A (molar mass 140 g mol^(-1)) was dissolved in 1000 g ...
Text Solution
|
- Vapour pressure of pure acetone and chloroform at 328 K are 741.8 mm H...
Text Solution
|
- Benzene and toluene form ideal solution over the entire range of compo...
Text Solution
|
- The air is a mixture of a number of gases. The major components are ox...
Text Solution
|
- Determine the amount of CaCl(2)(i=2.47) dissolved in 2.5 litre of wast...
Text Solution
|
- Determine the osmotic pressure of a solution prepared by dissolving 25...
Text Solution
|
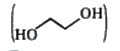 has polar - OH group and can form H - bond.
has polar - OH group and can form H - bond.