A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PROTEINS AND NUCLEIC ACIDS
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - II)|4 VideosPROTEINS AND NUCLEIC ACIDS
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - III)|3 VideosPROTEINS AND NUCLEIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET - 4|30 VideosPRINCIPLES OF METALLURGY
AAKASH SERIES|Exercise SUBJECTIVE EXERCISE-2 (LONG ANSWER QUESTIONS )|7 VideosREDOX REACTONS
AAKASH SERIES|Exercise Questions For Descriptive Answers|27 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-PROTEINS AND NUCLEIC ACIDS -LEVEL - II LECTURE SHEET (EXERCISE - I)
- The pH value of a solution in which a polar amino acid doesn't migrate...
Text Solution
|
- The structural feature which distinguishes proline from alpha - amino ...
Text Solution
|
- Which amino acid is achiral ?
Text Solution
|
- Which is not a true statement ?
Text Solution
|
- Which of the following structure represents the peptide chain ?
Text Solution
|
- All common amino acids except one react with cold nitrous acid (HNO(2)...
Text Solution
|
- The amino acid cysteine often forms a disulphide bond with another nea...
Text Solution
|
- Peptides on hydrolysis gives
Text Solution
|
- Peptides are composed of amino acids joined by amide bonds. Which of t...
Text Solution
|
- Which of the following is used in a colour test of amino acid ?
Text Solution
|
- Isoelectric point is
Text Solution
|
- Histidine has pK(a1)=1.8, pK(a2)=09.2 and pK(a3)=6.0. The isoelectric ...
Text Solution
|
- Glutamic acid, H(2)N-CH(CH(2)CH(2)COOH)COOH has pK(a1),(alpha-COOH)=2....
Text Solution
|
- Which of the following is the major solute species in a solution of ly...
Text Solution
|
- In an electric field, if an amino acid migrates towards cathode, the p...
Text Solution
|
- During the process of digestion, the proteins present in food material...
Text Solution
|
- The helical structure or a secondary structure of proteins is stabilis...
Text Solution
|
- Proteins give
Text Solution
|
- The destruction of the biological nature and activity of proteins by h...
Text Solution
|
- The primary structure of protein is based upon the
Text Solution
|
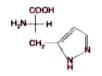 Histidine has `pK_(a1)=1.8, pK_(a2)=09.2` and `pK_(a3)=6.0`. The isoelectric point, PI of histidine is likely to be
Histidine has `pK_(a1)=1.8, pK_(a2)=09.2` and `pK_(a3)=6.0`. The isoelectric point, PI of histidine is likely to be