Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-3 (Single answer Questions)|15 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-3 (More than one correct answer questions)|4 VideosCARBOXYLIC ACIDS
AAKASH SERIES|Exercise PRACTICE SHEET-2 (Linked Compreshension Type Questions)|9 VideosCARBOHYDRATES
AAKASH SERIES|Exercise Practice Sheet-5|30 VideosCARBOXYLIC ACIDS AND DERIVATIVES
AAKASH SERIES|Exercise CONVERSIONS|19 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CARBOXYLIC ACIDS -PRACTICE SHEET-2 (Integer Type Questions)
- The minimum number of carbon atoms to be present to write an ester is
Text Solution
|
- Number of carbon atoms (minimum) be present for an acid to be opticall...
Text Solution
|
- Number of isomeric benzoic acids with formula C8 H8 O2 is
Text Solution
|
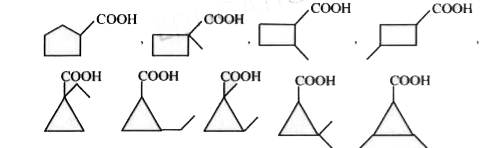
- Carboxylic acids containing carbocyclic ring, possible for C6 H(10)O2 ...
Text Solution
|
- Number of isomers possible for C4 H8 O2 (both acids & esters, excludin...
Text Solution
|
- Number of resonance structures for HCOO^- is
Text Solution
|
- Number of resonance structures possible for benzaldehyde
Text Solution
|