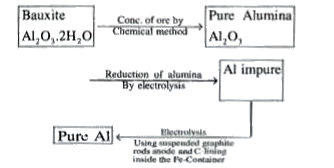
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
METALLURGY
AAKASH SERIES|Exercise LEVEL-II (LECTRURE SHEET) (EXERCISE-II) (PASSAGE-IV)|4 VideosMETALLURGY
AAKASH SERIES|Exercise LEVEL-II (LECTRURE SHEET) (EXERCISE-II) (PASSAGE-V)|3 VideosMETALLURGY
AAKASH SERIES|Exercise LEVEL-II (LECTRURE SHEET) (EXERCISE-II) (PASSAGE-II)|4 VideosIONIC EQUILLIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET ( ADVANCED))|11 VideosNITROGEN CONTAINING COMPOUNDS
AAKASH SERIES|Exercise Conversions|18 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-METALLURGY-LEVEL-II (LECTRURE SHEET) (EXERCISE-II) (PASSAGE-III)
- Extraction of Aluminiunt can be understood by : Electrolytic redu...
Text Solution
|
- Extraction of Aluminiunt can be understood by : Electrolytic redu...
Text Solution
|
- Extraction of Aluminiunt can be understood by : Electrolytic redu...
Text Solution
|
- Extraction of Aluminiunt can be understood by : Electrolytic redu...
Text Solution
|