The Killingham diagram for sing, magnesium and carbon converting into corresponding oxides is shown below :
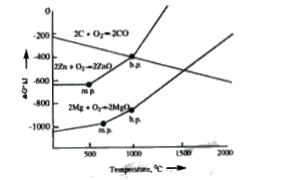
At `1000^(@)C` which reaction is spontaneous to a maximum extent ?
The Killingham diagram for sing, magnesium and carbon converting into corresponding oxides is shown below :

At `1000^(@)C` which reaction is spontaneous to a maximum extent ?

At `1000^(@)C` which reaction is spontaneous to a maximum extent ?
A
`MgO+C to Mg+CO`
B
`ZnO+ C to Zn+CO`
C
`MgO+Zn to Mg+ZnO`
D
`ZnO+Mg to MgO +Zn`
Text Solution
Verified by Experts
The correct Answer is:
D
Metal below in the graph reduces. Metal oxide into metal which is present above it.
The reaction `ZnO+Mg to MgO+Zn` will has most -ve net `DeltaG^(@)` value. So it is spontaneous maximum extent.
The reaction `ZnO+Mg to MgO+Zn` will has most -ve net `DeltaG^(@)` value. So it is spontaneous maximum extent.
Topper's Solved these Questions
METALLURGY
AAKASH SERIES|Exercise LEVEL-II (LECTRURE SHEET) (EXERCISE-II) (PASSAGE-VI)|3 VideosMETALLURGY
AAKASH SERIES|Exercise LEVEL-II (LECTRURE SHEET) (EXERCISE-II) (PASSAGE-VII)|2 VideosMETALLURGY
AAKASH SERIES|Exercise LEVEL-II (LECTRURE SHEET) (EXERCISE-II) (PASSAGE-IV)|4 VideosIONIC EQUILLIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (PRACTICE SHEET ( ADVANCED))|11 VideosNITROGEN CONTAINING COMPOUNDS
AAKASH SERIES|Exercise Conversions|18 Videos
Similar Questions
Explore conceptually related problems
The Killingham diagram for sing, magnesium and carbon converting into corresponding oxides is shown below : To make the following reduction process spontaneous, temperature should be
The Killingham diagram for sing, magnesium and carbon converting into corresponding oxides is shown below : At what temperature, zinc and carbon have equal affinity for oxygen?
The Ellingham diagram for a number of metallic sulphides is shown below Which sulphide occurs to minimum extent in nature
The Ellingham diagram for a number of metallic sulphides is shown below Formation of which of the sulphides is most spontaneous ?
The Ellingham diagram for a number of metallic sulphides is shown below Which of the following sulphides can not be reduced to metal by H_(2) at about 1000^(@)c
For a spontaneous reaction, the free energy change must be negative. DeltaG=DeltaH-TDeltaS is the enthalpy change during the reaction. T is absolute temperature, and AS is the change in entropy during the reaction. Consider a reaction such as the formation ofan oxide . M+O_(2) MO Dloxygen is used wp in the course of this reaction. Gases have a more random structure (less ordered) than liquid or solids consequently gases have a higher entropy than liquids and solids, in this reaction (entropy or randomness) decreases, hence is negative. Thus, the temperature is raised the DeltaS becomes more negative. Since, TDeltaS is subtracted in the equation, then SG becomes less negative. Thus, the free energy changes increases with the increase in temperature. The free energy changes that occur when one mole of common reactant in this case dioxygen) is we may e plotted graphically against temperature for a number of reactions of metals to their oxides. The following plot is called an Ellingham diagram for metal oxide. Understanding of Ellingham diagram is extremely important for the efficient extraction of metals. As per the Ellingham diagram of oxides which of the following conclusion is true?
In the below mentioned diagram four test-tubes A, B, C and D contains dil . HCl. Defferent metal granules are put inside the dil.HCI. In which test-tube the reaction is more vigorous at room temperature ?
For a spontaneous reaction, the free energy change must be negative. DeltaG=DeltaH-TDelaS is the enthalpy change during the reaction. T is absolute temperature, and AS is the change in entropy during the reaction. Consider a reaction such as the formation ofan oxide . M+O_(2) MO Dloxygen is used wp in the course of this reaction. Gases have a more random structure (less ordered) than liquid or solids consequently gases have a higher entropy than liquids and solids, in this reaction (entropy or randomness) decreases, hence is negative. Thus, the temperature is raised the DeltaS becomes more negative. Since, TDeltaS is subtracted in the equation, then SG becomes less negative. Thus, the free energy changes increases with the increase in temperature. The free energy changes that occur when one mole of common reactant in this case dioxygen) is we may e plotted graphically against temperature for a number of reactions of metals to their oxides. The following plot is called an Ellingham diagram for metal oxide. Understanding of Ellingham diagram is extremely important for the efficient extraction of metals. For the conversion of Ca(s) to CaO(s) which of the following represent the DeltaG vs T :
The products of bond breaking, shown below, are not stable, and cannot be isolated for prolonged study. Such species are referred to as reactive intermediate, and are belived to be transient intermediates in many reactions. The general structures and names of four such intermediates are, Charged Intermediates Uncharged Intermediates Carbocations (called carbonium ions in the older literature) are electrophiles and carbananions are nucleophiles. Carbenes have only a valence shell sextet of electrons and are therefore electron deficient. In this sense they are elecrophiles, but the non-bonding electron pair also gives carbenes nucleophilic character. As a rule, the electrophilic character dominates carbene reactivity. Carbon radicals have only seven valence electrons, and may be considered electron deficient, however, they do not in general bond to nucleophilic electron pair, so their chemistry exhibits differences from that of conventional electrophiles. Radical intermediates are often called free radicals. Intermediates are in general stabilised with conjugation, electron donating and electron with drawing groups. Which of the following is relatively an unstable intermediate compared to rest ?
The products of bond breaking, shown below, are not stable, and cannot be isolated for prolonged study. Such species are referred to as reactive intermediate, and are belived to be transient intermediates in many reactions. The general structures and names of four such intermediates are, Charged Intermediates Uncharged Intermediates Carbocations (called carbonium ions in the older literature) are electrophiles and carbananions are nucleophiles. Carbenes have only a valence shell sextet of electrons and are therefore electron deficient. In this sense they are elecrophiles, but the non-bonding electron pair also gives carbenes nucleophilic character. As a rule, the electrophilic character dominates carbene reactivity. Carbon radicals have only seven valence electrons, and may be considered electron deficient, however, they do not in general bond to nucleophilic electron pair, so their chemistry exhibits differences from that of conventional electrophiles. Radical intermediates are often called free radicals. Intermediates are in general stabilised with conjugation, electron donating and electron with drawing groups. Which of the following carbo cations is more stable?