Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (SINGLE OR MORE THAN ONE OPTION QUESTIONS)|16 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (LINKED COMPREHENSION TYPE QUESTIONS)|6 VideosCHEMICAL KINETICS
AAKASH SERIES|Exercise LEVEL-II(LECTURE SHEET EXERCISE-III MATCH THE FOLLOWING QUESTIONS)|3 VideosCHEMICAL KINETCS
AAKASH SERIES|Exercise EXERCISE - 3.2|45 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise Additional Practice Exercise|54 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL KINETICS-LEVEL-II(LECTURE SHEET EXERCISE-IV INTEGER ANSWER TYPE QUESTIONS)
- For the reaction Ato products the following dats is given for a partic...
Text Solution
|
- 50% of a reaction is completed in 20 minute and 75% is completed in 30...
Text Solution
|
- H(2)O(2)overset(1st"order")(to)H(2)O+1/2O(2). Pressure of O(2) is reco...
Text Solution
|
- H(2)O(2)toH(2)O+1/2O(2)(1storder) Time to time the H(2)O(2) solution...
Text Solution
|
- After one mole of A is consumed. What is the difference in moles of B ...
Text Solution
|
- In hypothetical reversible reaction underset((1M))(A)underset(t(1//...
Text Solution
|
- For a chemical reaction aAtoB,log[-(d[A])/(dt)]=log[(d[B])/(dt)]+0.3 t...
Text Solution
|
- The concentrationfo R in the reaction RtoP was measured as a function ...
Text Solution
|
- An organic compound undergoes first order decomposition. The time take...
Text Solution
|
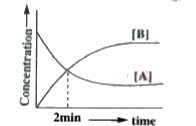
- For a first order reaction A((g))hArr3B((g)) the concentration verses ...
Text Solution
|