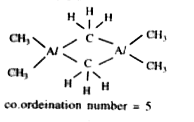
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) LEVEL - I (MAIN)|12 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) LEVEL - II (ADVANCED)|30 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II) LEVEL - I (MAIN)|12 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II (PRACTICE SHEET ADVANCE)) (Integer Type Questions)|3 VideosELEMENTS OF CARBON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (Recent AIPMT/NEET Questions) |10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELEMENTS OF BORON FAMILY-LECTURE SHEET (EXERCISE - II) LEVEL - II (ADVANCED)
- Which is pure basic oxide
Text Solution
|
- The number of sigma and pi bonds present in inorganic benzene
Text Solution
|
- B(OH)(3)+NaOH hArr NaBO(2)+Na[B(OH)(4)]+H(2)O How can this reactio...
Text Solution
|
- Boric acid is prepared from borax by the action of
Text Solution
|
- Select the correct statements about diborane
Text Solution
|
- Al(2)(SO(4))(3)+NH(4)OH rarr X,X is
Text Solution
|
- Which of the following reaction(s) is/are involved in thermit process ...
Text Solution
|
- In the reaction 2X+B(2)H(6)rarr [BH(2)X(2)]^(+)[BH(4)]^(-) the ...
Text Solution
|
- Which of the following oxides are basic ?
Text Solution
|
- Alumina is
Text Solution
|
- Potash alum is used as a
Text Solution
|
- Boranes have general formula
Text Solution
|
- Hydrated AlCl(3) is used as
Text Solution
|
- Boron reacts with oxygen at 700^(@)C to give (A). Compound (A) reacts ...
Text Solution
|
- Boron reacts with oxygen at 700^(@)C to give (A). Compound (A) reacts ...
Text Solution
|
- Boron reacts with oxygen at 700^(@)C to give (A). Compound (A) reacts ...
Text Solution
|
- {:(,"Column - I",,"Column - II"),((A),H(3)BO(3),(P),"Hydrogen bonds"),...
Text Solution
|
- In solid conundrum the number of oxygen atoms coordinate to aluminium ...
Text Solution
|
- Total number of molecules having three centered two e^(-) bonds among ...
Text Solution
|
- Tri alkyl aluminium molecules exists as dimer which contains 3 centere...
Text Solution
|