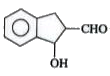
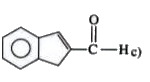
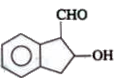
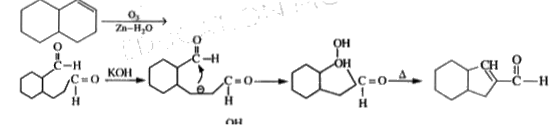
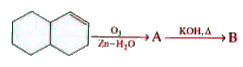A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
AAKASH SERIES|Exercise LEVEL-II LECTURE SHEET (EXERCISE-II)|28 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise LEVEL-II LECTURE SHEET (EXERCISE-III)|11 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise LEVEL-I(EXERCISE-II)|59 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise ETHERS (OBJECTIVE EXERCISE-4)|50 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)|19 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALDEHYDES AND KETONES-LEVEL-II LECTURE SHEET (EXERCISE-I)
- Which of the following compounds contains most acidic hydrogen ...
Text Solution
|
- Which hydrogen of the given compound is least acidic in nature ?
Text Solution
|
- Compound B is
Text Solution
|
- Compound (Y) is
Text Solution
|
- product product will be
Text Solution
|
- In the given reaction X will be
Text Solution
|
- Which of the following compound would be most reactive for per...
Text Solution
|
- Cinnamic acid from benzaldehyde would be prepared by which of ...
Text Solution
|
- Prredict the major product of reaction Product (A) is
Text Solution
|
- compound A and B can be differentiated by
Text Solution
|
- these compounds can be differentiated by :
Text Solution
|
- Find out final product (X) :
Text Solution
|
- find major product (X)
Text Solution
|
- Major product (X) :
Text Solution
|
- Product (X ) of reaction is
Text Solution
|
- y product 'Y' is
Text Solution
|
- final product Y is
Text Solution
|
- Major product (X) . Find out (X ) of the reaction
Text Solution
|
- 'Y' will be
Text Solution
|
- product 'A' will be
Text Solution
|