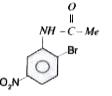
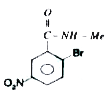
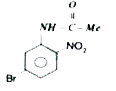
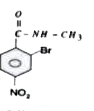
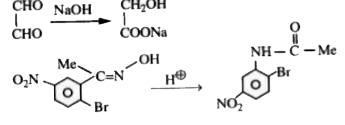
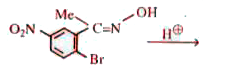A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
AAKASH SERIES|Exercise PRACTICE SHEET-2|30 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise PRACTICE SHEET-3|34 VideosALDEHYDES AND KETONES
AAKASH SERIES|Exercise LEVEL-II LECTURE SHEET (EXERCISE-V)|16 VideosALCOHOLS, PHENOLS AND ETHERS
AAKASH SERIES|Exercise ETHERS (OBJECTIVE EXERCISE-4)|50 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)|19 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ALDEHYDES AND KETONES-PRACTICE SHEET-1
- In the reaction sequence : C6H5 COCH3 overset( NH2 OH //H^+) to (X )...
Text Solution
|
- Which statements is/are correct ?
Text Solution
|
- Which of the following structures represent an acetal
Text Solution
|
- The product obtained in which of the following reactions is same
Text Solution
|
- Which of the following reagents is useful to distinguish between propo...
Text Solution
|
- Aldehydes undergo disproportionation reaction in presence of aqueous N...
Text Solution
|
- Aldehydes undergo disproportionation reaction in presence of aqueous N...
Text Solution
|
- Aldehydes undergo disproportionation reaction in presence of aqueous N...
Text Solution
|
- Aldehydes and ketones react with NH2 OH under slightly acidic conditio...
Text Solution
|
- Aldehydes and ketones react with NH2 OH under slightly acidic conditio...
Text Solution
|
- Aldehydes and ketones react with NH2 OH under slightly acidic conditio...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Butanone & propanone were allowed to react is presence of OH,total ald...
Text Solution
|
- Among the following, the compounds which gives iodoform reaction with ...
Text Solution
|
- The number of aldehydes with formula C5 H(10) O is
Text Solution
|
- The number of ketone isomers containing benzene ring with formula C9 H...
Text Solution
|
- The disubstituted aldehyde derivatives with formula C9 H(1) O
Text Solution
|
- Total aldol products of reaction between CH3 CHO & CH3 CH2 CHO is
Text Solution
|
- Total number of Grignard reagent molecules which can react with
Text Solution
|