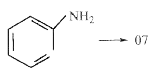Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (Single answer questions)|10 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (More than one correct answer questions)|5 VideosAMINES AND AZO COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 1 (Linked Comprehension type questions (Passage -II))|3 VideosALKYL AND ARYL HALIDES
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)|19 VideosAMINES AND DIAZONIUM SLATS
AAKASH SERIES|Exercise PRACTICE EXERCISE|25 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-AMINES AND AZO COMPOUNDS -PRACTICE SHEET - 1 (Matching Type Questions)
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- The number of compounds which contain at least one basic nitrogen is
Text Solution
|
- The number of atoms which are sp^(2) hybridised in aniline is
Text Solution
|
- The number of primary amines possible for C(4)H(9)N (exclude stereoiso...
Text Solution
|
- The number of primary amines possible for C(4)H(9)N (exclude stereoiso...
Text Solution
|
- The number of p-disubstituted benzene derivations of C(9)H(13)N is
Text Solution
|
- The number of amines which behave like atiphatic amines from the follo...
Text Solution
|
- Number of tertiary amines possible fo C(6)H(5)N (excluding stereoisome...
Text Solution
|