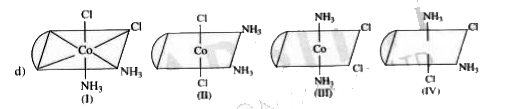
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise LEVEL-II LECTURE SHEET EXERCISE-II (Linked Comprehension type questions ) Passage-I|3 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise LEVEL-II LECTURE SHEET EXERCISE-II (Linked Comprehension type questions ) Passage-II|3 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise LEVEL-I (EXERCISE-II)|52 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (PRACTICE SHEET -2 (INTEGER ANSWER TYPE QUESTIONS))|7 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CO-ORDINATION COMPOUNDS-LEVEL-II LECTURE SHEET EXERCISE-I (Single & One or more than One Correct Answers )
- Which of the following statement(s) is (are) correct?
Text Solution
|
- For [Fe(CN)(6)]^(4-).[Ni(CN)(4)]^(2-)and[Ni(CO)(4)]. Correct statement
Text Solution
|
- Which have octahedral shape (d^(2)sp^(3)) hybridisation of central ato...
Text Solution
|
- Which possesses tetrahedral shape (sp^(3)- hybridisation of central at...
Text Solution
|
- Which of the following complexes is a chelate ?
Text Solution
|
- A complex shown below can exhibit :
Text Solution
|
- Which of the following compounds can exhibit geometrical isomerism?
Text Solution
|
- Which of the following isomerisms are exhibited by [Cr(NH(3))(2)(OH)(2...
Text Solution
|
- Which of the following statements is not true about the complex ion [C...
Text Solution
|
- Which of the following statement(s) is/are incorrect?
Text Solution
|
- Which of the following statements is/are true ?
Text Solution
|
- Which of the following statement is true about the complex [CrCl(3)(OH...
Text Solution
|
- Which of the following pairs show coordination isomerism ?
Text Solution
|
- A metal comples having composition Cr(NH(3))(4)CI(2)Br has been isolat...
Text Solution
|
- In poly nuclear metal carbonyls, the hybridisation of 'C' in terminal ...
Text Solution
|
- Which is high spin complex :
Text Solution
|
- A d-block element forms octahedral complex but its magnetic moment rem...
Text Solution
|
- For which of the following d^(n) configuration of octahedral complex(e...
Text Solution
|
- Select the correct statement
Text Solution
|
- Which one of the following statement (s) is/are false ?
Text Solution
|