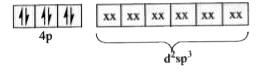
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 4 ( Single or more than one option questions C.E.T., Stability, Applications:|16 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 4 ( Linked Comprehension type questions) Passage-I|3 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 3 ( Match the following questions)|2 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (PRACTICE SHEET -2 (INTEGER ANSWER TYPE QUESTIONS))|7 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CO-ORDINATION COMPOUNDS-PRACTICE SHEET - 3 (Integer answer type Questions)
- Ni^(+2) ion forms a searlet red ppt with DMG. It is a …………. Memberd ri...
Text Solution
|
- The CN of copper in complex formed by adding excess of NH(3) to CuSO(...
Text Solution
|
- The ratio of CN and OS of x in [x(NH(3))(5)SO(4)]Cl is
Text Solution
|
- Cu^(+2) and and 'en gives a chelate complex. It is ... Membered
Text Solution
|
- The number of octahedral voids in a unit cell of cubic close packed st...
Text Solution
|